
ORIGINAL ARTICLE
Fruit peels as sources of bioactive compounds with antioxidant and antimicrobial properties
Cáscaras de frutas como fuentes de compuestos bioactivos con propiedades antioxidantes y antimicrobianas
Miguel A. Aguilar-Méndez 1, Martha P. Campos-Arias 1, Cinthya N. Quiroz-Reyes 1, Elba Ronquillo-de Jesús 2, Miguel A. Cruz-Hernández 3
1 Instituto Politécnico Nacional. Centro de Investigación en Ciencia Aplicada y Tecnología Avanzada. Legaria 694. C. P. 11500. Ciudad de México. México. * maguilarme@ipn.mx
2 Universidad Politécnica de Francisco I. Madero Dirección de Ingeniería Agroindustrial. Domicilio conocido. C. P. 42660. Tepatepec. Hidalgo. México.
3 Colegio de Postgraduados Campus Montecillo. Instituto de Edafología. Carretera México-Texcoco km 36.5. C. P. 56230. Texcoco. Estado de México. México
Originales: Recepción: 08/10/2018 - Aceptación: 31/08/2019
ABSTRACT
Recently, a major interest in searching for phytochemicals with nutritional and pharmaceutical purposes has arisen. In this regard, it is known that polyphenols present antioxidant properties as well as an inhibitory effect against some kinds of microorganisms. The aim of this study was to obtain aqueous-ethanolic extracts from peels of avocado, cocoa bean, coconut and cactus pear by ultrasound-assisted extraction. The extracts were characterized in terms of phenolics (Folin-Ciocalteu reagent), antioxidant potential (ferric reducing/antioxidant power assay), radical-scavenging ability (2,2-diphenyl-2-picrylhydrazyl free radical assay), and antimicrobial activity against Staphylococcus aureus, Shigella dysenteriae and Candida albicans (disk diffusion test). The results revealed that the avocado peel extract had the highest phenol content (36.5 mg EAG g-1 dry weight), the highest antioxidant activity (141.2 mME Trolox g-1 dry weight) and the lowest IC50 value (59 ppm). Furthermore, avocado and coconut peels demonstrated an inhibitory effect against the tested microorganisms.
Keywords: Fruit peel; Phenolic compound; Antioxidant activity; Antimicrobial activity
RESUMEN
En los últimos años se ha producido un gran interés en la búsqueda de fitoquímicos con fines nutricionales y farmacéuticos. A este respecto, se sabe que los polifenoles presentan propiedades antioxidantes, así como un efecto inhibidor contra algunos tipos de microorganismos. En este estudio se obtuvieron extractos acuoetanólicos de cáscaras de aguacate, cacao, coco y tuna mediante extracción asistida por ultrasonido. Los extractos se caracterizaron en términos de fenoles (reactivo de Folin-Ciocalteu), potencial antioxidante (prueba del poder reductor férrico/antioxidante), capacidad secuestradora de radicales (prueba del radical libre 2,2-difenil-1-picrilhidracilo) y actividad antimicrobiana contra Staphylococcus aureus, Shigella dysenteriae y Candida albicans (método de difusión con discos). Los resultados revelaron que el extracto de cáscara de aguacate presentó el contenido más alto de fenoles (36,5 mg EAG/g materia seca), la mayor actividad antioxidante (141,2 mME Trolox/g de materia seca) y el valor más bajo de IC50 (59 ppm). Además, las cáscaras de aguacate y coco demostraron un efecto inhibitorio contra los microorganismos testados.
Palabras clave: Cáscara de fruta; Compuesto fenólico; Actividad antioxidante; Actividad antimicrobiana
INTRODUCTION
Traditionally, plants and fruits have been used for obtaining compounds with biological activity (21, 22, 25). Specifically, fruit hulls or peels have been listed as potential sources of compounds with antioxidant and antimicrobial properties. This part of the fruit is non-edible material discarded during the manufacturing processes (15). Although they are typically considered waste material, it has been reported that several of these materials are promising sources of valuable components, such as phenolic compounds (polyphenols, flavonoids and tannins), and other bioactive components (8). Peel of many fruits has already been used for the extraction of phenolic compounds, for example kinnow (27), mango (1), melon (20), orange (11, 17), pear (15), pomegranate (29, 32), among others. It has even been reported that some peels obtained from fruits like apple, hawthorn, and pomegranate present a significantly greater amount of phenolic compounds in comparison with the pulp.
Polyphenols are secondary metabolites that play essential roles in plant physiology and have beneficial properties for human health, mainly as antioxidants and antimicrobials (3, 4, 6). Polyphenolic compounds display antioxidant activity through different mechanisms, in particular by free radical scavenging and by chelation of metal ions (12). On the other hand, the antimicrobial properties of polyphenols are related to their structural configuration, being the hydroxyl (-OH) group the responsible for inhibitory action (8). Recently, considerable interest in the use of natural compounds with antioxidant and antimicrobial activity has arisen, not only for food preservation and shelf life improvement, but also for increasing stability of fats and oils, and for controlling microbial diseases in both humans and plants (7, 13, 19).
Fruits like avocado, cocoa, coconut and cactus pear are native and/or major crops in Mexico. However, little has been studied about the use of by-products like the peel of these fruits. Therefore, the aim of this study was to evaluate the antioxidant and antimicrobial activity of aqueous ethanolic extracts obtained from peels of avocado, cocoa, coconut and cactus pear.
MATERIALS AND METHODS
Chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, Folin-Ciocalteu reagent, Tris-HCl Buffer, 2,4,6-Tris (2-pyridyl)-s-triazine (TPTZ) and ferric chloride hexahydrate were purchased from Sigma-Aldrich (USA). Acetic acid, ascorbic acid, sodium acetate, sodium chloride and sulphuric acid were obtained from JTBaker (Mexico). Mueller Hinton agar (Bioxon) and chloramphenicol (Sophia Laboratories, Mexico) were used for microbiological analysis. All the solvents used were analytical reagent grade.
Preparation of plant material
Avocado (Hass), cocoa (Forastero), coconut (Acapulco) and cactus pear (San Martin) fruits were purchased at a local market in Mexico City. The fruits were washed with water and sanitized with a solution of sodium hypochlorite 1%. Subsequently, their peels were removed and dried in an oven at 40°C for 48 h. In the cases of cocoa and coconut, the inner shell (endocarp) was selected. Finally, the peels were pulverized in a disc mill (Model 148-2, The Bauer Bros Co., USA) and stored.
Ultrasound-assisted extraction
In all cases, extracts were obtained using a sample-solvent ratio of 1:20. Aqueous ethanolic extractions (70:30) were performed under sonication (25 kHz) for 30 min in an ultrasonic bath (TI-H-5, Elma, Germany). Subsequently, the extracts were centrifuged at 1750 rpm, filtered (Whatman no. 1), and concentrated (35°C) in a rotary evaporator (RE-500, Yamato, Japan). Finally, all samples were dried in a vacuum oven (Precision, Thelco, USA) at 35°C.
Quantification of total phenols
Total phenolic content was calculated from the reduction capacity of Folin- Ciocalteu using gallic acid as a standard (5). A 20-μL sample volume was added to 1.4 mL of distilled water, followed by 100 μL of Folin-Ciocalteu reagent. The final solution was allowed to stand for 5 min at room temperature. Subsequently, 300 μL of a sodium carbonate solution was added (20% w/v). After resting for 90 min in a dark room, absorbance was determined at a wavelength of 760 nm on a Cary 50 (Varian, USA) spectrophotometer. Results were expressed as mg gallic acid equivalents∙g-1 dry weight.
Ferric-Reducing Antioxidant Power (FRAP) Assay
Antioxidant capacity was determined using the FRAP (Ferric Reduction Antioxidant Power) test, modified (16). This assay determines the antioxidant capacity of the polyphenols to reduce TPTZ-Fe3 + complex. The FRAP reagent is prepared by mixing 25 mL of a 0.3 M acetate buffer (pH 3.6), 2.5 mL TPTZ solution (0.01 M) and 2.5 mL of a solution of FeCl3∙6H2O (0.02M) at 37°C. A sample of 150-μL extract was mixed with 2850 μL of FRAP solution and allowed to stand for 30 minutes in the dark.
Absorbance was recorded at a wavelength of 593 nm. The results were reported in mM Trolox eq∙g-1 dry weight.
DPPH Radical Scavenging Ability
The antiradical capacity was determined by the DPPH (2,2-diphenyl- 1-picrylhydrazyl) assay (22). A 2-mL aliquot of extract was mixed with 500 μL of 0.1M Tris-HCl buffer by vortex mixing for 5 seconds. To this solution, 2 mL of a 200-μM DPPH solution were added. After 30 minutes, absorbance was determined at 517 nm. The control sample consisted of a solution of ascorbic acid. The percentage of DPPH reduction was calculated using (eq. 1).

The EC50 value was determined from the data contained in the DPPH reduction effect against the extract concentration graph.
Antimicrobial activity
The antimicrobial activity was evaluated in vitro by disk diffusion assay, modified (28). Extracts were dissolved in distilled water at a concentration of 200 mg mL-1 to evaluate their activity against Staphylococcus aureus, Candida albicans and Shigella dysenteriae. A standardized suspension of the microorganisms was spread on Mueller Hinton agar culture medium using swabs. Paper disks (6mm diameter) were impregnated with 20 μL of extract and placed on the inoculated agar. The petri dishes were incubated at 37°C for 24 h. The antimicrobial activity was evaluated by measuring the zone of inhibition test against microorganisms. Chloramphenicol and distilled water were used as positive and negative controls, respectively.
HPLC analysis
The two extracts with the best antioxidant and antimicrobial properties were analyzed by HPLC for phenolic identification. HPLC-analyses were carried out in an Agilent 1200 chromatograph (Agilent Technologies, Germany) equipped with a multiple wavelength detector. Separations were conducted on a Zorbax Eclipse XDB-C18 (3.5 μm, 100x4.6 mm). The mobile phase consisted of water/formic acid (99.9/0.1) as eluent A and methanol/acetonitrile (50/50) as eluent B. The system was run with a gradient program: 13- 17% B for 21 min, 17-23% B for 14 min and 23-33% for 5 min. The Column temperature was set at 30°C, flow rate was 300 μL per min and the injection volume was 5 μL. Samples were previously dissolved in demineralized water/ethanol and filtered through a 0.45 μm membrane filter. Peaks of ascorbic acid, oxalic acid, ferulic acid, gallic acid, (+)- catechin, (-)-epicatechin, procyanidin B1 and procyanidin B2 were identified by comparing the retention times of samples with those of standards. Chromatograms were recorded at 280 nm (26).
Statistical analysis
All the analyses were performed in triplicate, and the results were analyzed using ANOVA (Design Expert 8). Differences between means were detected by the Duncan multiple range test. Differences were considered significant at a significance level (α) of 0.05.
RESULTS AND DISCUSSION
Total phenol content
Many phenolic compounds found in fruits and vegetables have generated much interest due to their antioxidant potential. The total phenolic contents studied in this work are presented in figure 1, which shows that avocado peel has the highest content of total phenols (36.5±0.5 mg GAE∙g-1 dry weight), followed by coconut (13.6±0.5 mg∙GAE g-1 dry weight).

Figure 1. Total phenolic content of fruit peels extracts.
Figura 1. Contenido fenólico total de extractos de cáscaras de fruta.
However, no differences were detected between mean values obtained for cocoa and cactus pear extracts (P > 0.05). Phenolic contentsfound in this study resulted lower than those reported for mango peel (54-109 mg GAE∙g-1 dry weight) (1) and pomegranate (55-89 mg GAE∙g-1 dry weight) (28), but higher than those reported for peels from different pear varieties (2.6-11.2 mg GAE∙g-1 dry weight) (15) and gac fruit (2.31-2.80 mg GAE∙g-1 dry weight) (14).
Antioxidant capacity
Antioxidant activity mainly rests on redox properties of various compounds, that act as reducing agents or hydrogen atom donors (23). Phenolic compounds and pigments are the main groups of compounds that contribute to antioxidant activity in vegetables, fruits, cereals and other plant materials.
According to figure 2, the avocado peel extract showed the highest antioxidant activity (141.23 mM Trolox equivalents∙g-1 dry weight), as measured by the reduction of Fe3+ to Fe2+, statistically different from the values obtained for the cocoa, coconut and cactus pear extracts (P < 0.05).

Figure 2. Antioxidant capacity of fruit peels extracts measured by FRAP. Values are expressed as mean±sd (n = 3).
Figura 2. Capacidad antioxidante de extractos de cáscaras de frutas medida mediante FRAP. Valores expresados como media±de (n = 3).
These results could be positively correlated with total phenol content (R2 = 0.98), indicating that the higher the phenolic content, the higher antioxidant activity. Nedamani et al. (2014) also reported a substantial relationship between total phenols and antioxidant activity in extracts of rosemary leaves and oak fruit.
Antiradical activity
The DPPH radical is a stable radical widely used to determine the ability of plant extracts acting as free radical scavengers or hydrogen donors. Figure 3 (page 366), shows radical inhibition vs concentration of the tested extracts.
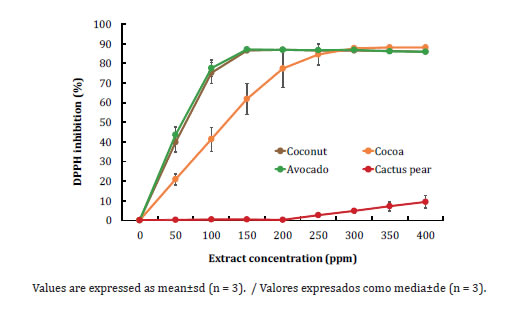
Figure 3. DPPH radical scavenging activity of fruit peels extracts.
Figura 3. Actividad secuestradora del radical DPPH de extractos de cáscaras de frutas.
The avocado and coconut extracts showed inhibitory activity that increased rapidly in the range of 0-100 ppm, reaching inhibition values of up to 75% and remaining almost constant at higher concentrations. Meanwhile, cocoa and cactus pear extracts showed lower inhibitory response against the DPPH radical.
The EC50 is defined as the amount of antioxidant required to reduce the initial DPPH radical concentration by 50%. The lower the EC50 value, the greater the DPPH radical scavenging activity of the extracts (31).
Table 1 shows the EC50 values for the different extracts.
Table 1. EC50 values from fruit peels extracts.
Tabla 1. Valores de EC50 de extractos de cáscaras de frutas.

The avocado peel extract had the lowest value, whereas it was not possible to determine an EC50 value for the cactus pear extract because of its poor activity as DPPH radical scavenger.
Antimicrobial activity
Table 2 (page 367), shows the inhibition zone diameters caused by the extracts tested against microorganisms.
Table 2. Antimicrobial activity from fruit peels extracts.
Tabla 2. Actividad antimicrobiana de extractos de cáscaras de frutas.
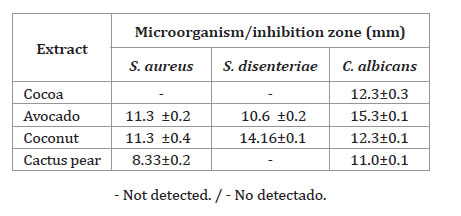
It can be observed that the avocado and coconut peel extracts showed the highest inhibition values against S. aureus, S. dysenteriae and C. albicans.
In the case of cactus pear peel extract, no inhibition was observed against S. dysenteriae, while the cocoa extract only showed antimicrobial activity against C. albicans. It is possible that the differences in antimicrobial activity among extracts are due to variations in phenolic content, as well as microorganism sensitivity.
According to several authors, the antimicrobial activity of phenolic compounds involves the reaction of phenols with cell membrane proteins and/or sulfhydryl protein groups, leading to bacterial death by precipitation of membrane proteins and inhibition of some enzymes (8, 9).
It should be noted that the extracts with higher antioxidant activity were also more active against the tested microorganisms, being this fact more evident for avocado extract. Jimenez et al. (2011) also reported a correlation between antioxidant capacity and antimicrobial activity in black cherry extracts (Prunus serotina subsp capuli).
HPLC
Typical chromatograms of avocado and coconut peel extracts obtained by sonication are shown in figure 4 (page 368).
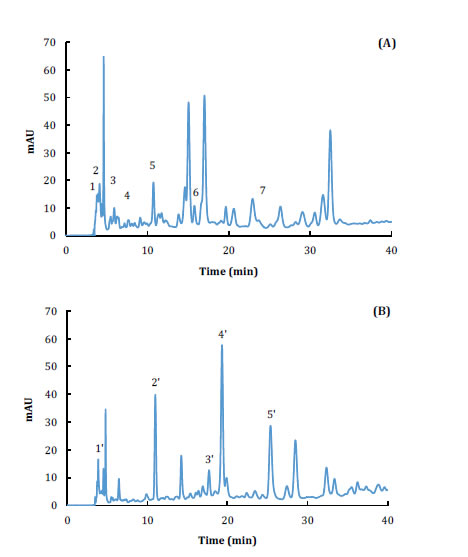
Figure 4. Chromatograms of coconut peel extract (A): (1) oxalic acid, (2) ascorbic acid, (3) ferulic acid, (4) gallic acid, (5 ) procyanidin B1, (6) (+)-catechin, (7) caffeic acid; and avocado peel extract (B): (1') ascorbic acid, (2') procyanidin B1, (3') (+)-catechin, (4') procyanidin B2, (5') (-)- epicatechin.
Figura 4. Cromatogramas de extracto de cáscara de coco (A): (1) ácido oxálico, (2) ácido ascórbico, (3) ácido ferúlico, (4) ácido gálico, (5) procianidina B1, (6) (+)-catequina, (7) ácido cafeico; y de extracto de cáscara de aguacate (B): (1') ácido ascórbico, (2') procianidina B1, (3') (+)-catequina, (4') procianidina B2, (5') (-)-epicatequina.
The presence of oxalic acid, ascorbic acid, ferulic acid, gallic acid, procyanidin B1, (+)-catechin and caffeic acid were identified in the coconut chromatogram (figure 4-A, page 368). Meanwhile, ascorbic acid, procyanidin B1, (+)-catechin, procyanidin B2 and (-)-epicatechin were confirmed in the avocado extract (figure 4-B, page 368). Flavonols, which were present in both extracts (catechin and epicatechin), could have an important role in the observed antimicrobial properties. Alonso-Esteban et al. (2019) reported important antimicrobial properties of (+)-catechin and (-)-epicatechin against B. cereus, L. monocytogenes, E. faecalis, E. coli, and S. typhymurium. Catechins can increase the content of reactive oxygen species in cells and cause endogenous oxidative stress in bacteria such as E. coli (18).
CONCLUSIONS
Bioactive compounds from avocado, cocoa, coconut, and cactus pear peels were obtained by ultrasound-assisted extraction. The aqueous ethanolic extracts from avocado peel presented the highest phenolic content and the best antioxidant and antiradical activities.
The results showed positive relationships between total phenolic content and antioxidant activity in all extracts. Furthermore, avocado and coconut peels extracts presented important inhibiting action against S. aureus, S. dysenteriae and C. albicans. We conclude that fruit by-products such as peels, could represent an important source of bioactive compounds with antioxidant and antimicrobial properties, and potential use in the pharmaceutical and food industries.
1. Ajila, C. M.; Naidu, K. A.; Bhat, S. G.; Prasada Rao, U. J. S. 2007. Bioactive compounds and antioxidant potential of mango peel extract. Food Chemistry. 105(3): 982-988.
2. Alonso-Esteban, J. I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R. C.; Torija-Isasa, E.; Sánchez-Mata, M. C.; Ferreira, I. C. F. R. 2019. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) seeds. Industrial Crops & Products. 134: 154-159.
3. Al-Zoreky, N. S. 2009. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International Journal of Food Microbiology. 134(3): 244-248.
4. Bubonja-Sonje, M.; Giacometti, J.; Abram, M. 2011. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chemistry. 127(4): 1821-1827.
5. Cardenas-Sandoval, B. A.; López-Laredo, A. R.; Martínez-Bonfil, B. P.; Bermúdez-Torres, K.; Trejo-Tapia, G. 2012. Advances in the phytochemistry of Cuphea aequipetala, C. aequipetala var. hispida and C. lanceolata: extraction and quantification of phenolic compounds and antioxidant Activity. Revista Mexicana de Ingeniería Química. 11(3): 401-413.
6. Daglia, M. 2012. Polyphenols as antimicrobial agents. Current opinion in Biotechnology. 23(2): 174-181.
7. González-Gómez, D.; Cardoso, V.; Bohoyo, D.; Ayuso, M. C.; Delgado-Adamez, J. 2014. Application of experimental design and response surface methodology to optimize the procedure to obtain a bactericide and highly antioxidant aqueous extract from orange peels. Food Control. 35(1): 252-259.
8. Gyawali, R.; Ibrahim, S. A. 2014. Natural products as antimicrobial agents. Food Control. 46: 412-429.
9. Ismail, T.; Sestili, P.; Akhtar, S. 2012. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. Journal of Ethnopharmacology. 143(2): 397-405.
10. Jiménez, M.; Castillo, I.; Azuara, E.; Beristain, C. I. 2011. Antioxidant and antimicrobial activity of capulin (Prunus serotina subsp capuli) extracts. Revista Mexicana de Ingeniería Química. 10(1): 29-37.
11. Khan, M. K.; Abert-Vian, M.; Fabiano-Tixier, A. S.; Dangles, O.; Chemat, F. 2010. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chemistry. 119(2): 851-858.
12. Khonkarn, R.; Okonogi, S.; Ampasavate, C.; Anuchapreeda, S. 2010. Investigation of fruit peel extracts as sources for compounds with antioxidant and antiproliferative activities against human cell lines. Food and Chemical Toxicology. 48(8-9): 2122-2129.
13. Kossah, R.; Zhang, H.; Chen, W. 2011. Antimicrobial and antioxidant activities of chinese sumac (Rhus typhina L.) fruit extract. Food Control. 22(1): 128-132.
14. Kubola, J.; Siriamornpun, S. 2011. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of thai gac (Momordica cochinchinensis Spreng). Food Chemistry. 127(3): 1138-1145.
15. Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. 2014. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chemistry. 152(1): 531-538.
16. Lim, Y. S.; Hui, L. S. S.; Chin, T. B. 2013. Antioxidant capacity and antibacterial activity of different parts of mangosteen (Garcinia mangostana Linn.) extracts. Fruits. 68(6): 483-489.
17. Luengo, E.; Alvarez, I.; Raso, J. 2013. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innovative Food Science & Emerging Technologies. 17: 79-84.
18. Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. 2019. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control. 106: 106712.
19. Majhenic, L.; Kerget, M. S.; Knez, Z. 2007. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chemistry. 104(3): 1258-1268.
20. Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. 2017. Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chemistry. 221(15): 1691-1697.
21. Mansilla, J. T.; Tarcaya, V. P.; Cufre, I. M.; Fabrizio, M. C.; Wright, E. R.; Broussalis, A. M.; Rivera, M. C. 2018. Control of Rhizoctonia solani with extracts from Ovidia andina. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 50(2): 355-368.
22. Márquez, D. M.; Galeano, E.; Martínez, A. 2003. Productos naturales con actividad antimicrobiana. Parte I. Vitae. 10(2): 61-71.
23. Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. 2014. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. Journal of Taibah University for Science. 8(3): 216-224.
24. Nedamani, E. R.; Mahoonak, A. S.; Ghorbani, M.; Kashaninejad, M. 2014. Antioxidant properties of individual vs. combined extracts of rosemary leaves and oak fruit. Journal of Agricultural Science and Technology. 16: 1575-1586.
25. Preciado-Rangel, P.; Gaucín-Delgado, J. M.; Salas-Pérez, L.; Sánchez Chavez, E.; Mendoza- Vllarreal, R.; Rodríguez Ortiz, J. C. 2018. The effect of citric acid on the phenolic compounds, flavonoids and antioxidant capacity of wheat sprouts. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 50(2): 119-127.
26. Quiroz-Reyes, C. N.; Aguilar-Méndez, M. A.; Ramírez-Ortiz, M. E.; Ronquillo-de Jesús, E. 2013. Comparative study of ultrasound and maceration techniques for the extraction of polyphenols from cocoa beans (Theobroma cacao L.). Revista Mexicana de Ingeniería Química. 12(1): 11-18.
27. Safdar, M. N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A. A. 2017. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. Journal of Food and Drug Analysis. 25(3): 488-500.
28. Saravanan, S.; Parimelazhagan, T. 2014. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Science and Human Wellness. 3(2): 56-64.
29. Sood, A.; Gupta, M. 2015. Extraction process optimization for bioactive compounds in pomegranate peel. Food Bioscience. 12: 100-106.
30. Tabaraki, R.; Heidarizadi, E.; Benvidi, A. 2012. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Separation and Purification Technology. 98: 16-23.
31. Wootton-Beard, P. C.; Ryan, L. 2011. Improving public health?: The role of antioxidant-rich fruit and vegetable beverages. Food Research International. 44(10): 3135-3148.
32. Yasoubi, P.; Barzegar, M.; Sahari, M. A.; Azizi, M. H. 2007. Total phenolic contents and antioxidant activity of pomegranate (Punica granatum L.) peel extracts. Journal of Agricultural Science and Technology. 9: 35-42.
ACKNOWLEDGEMENTS
This work was financially supported by SIP-IPN (project: 20140034). M. P. Campos-Arias thanks COFAA-IPN and CONACYT for the scholarships received.
Authors are also grateful for the technical assistance and facilities provided by Dra. Adriana Cuadros and Dr. Jorge Yañez, respectively.