Influence of Apis mellifera in-hive conditions on germination capacity of rapeseed pollen (Brassica napus)
DOI:
https://doi.org/10.48162/rev.39.148Palabras clave:
colza, germinabilidad, abeja melífera, polinizaciónResumen
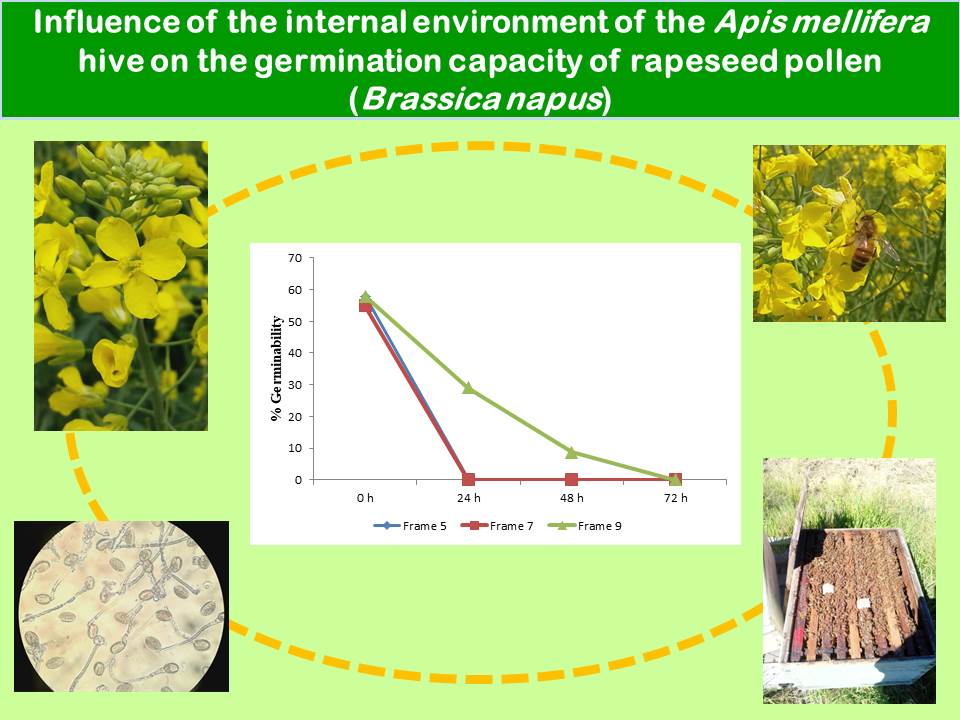
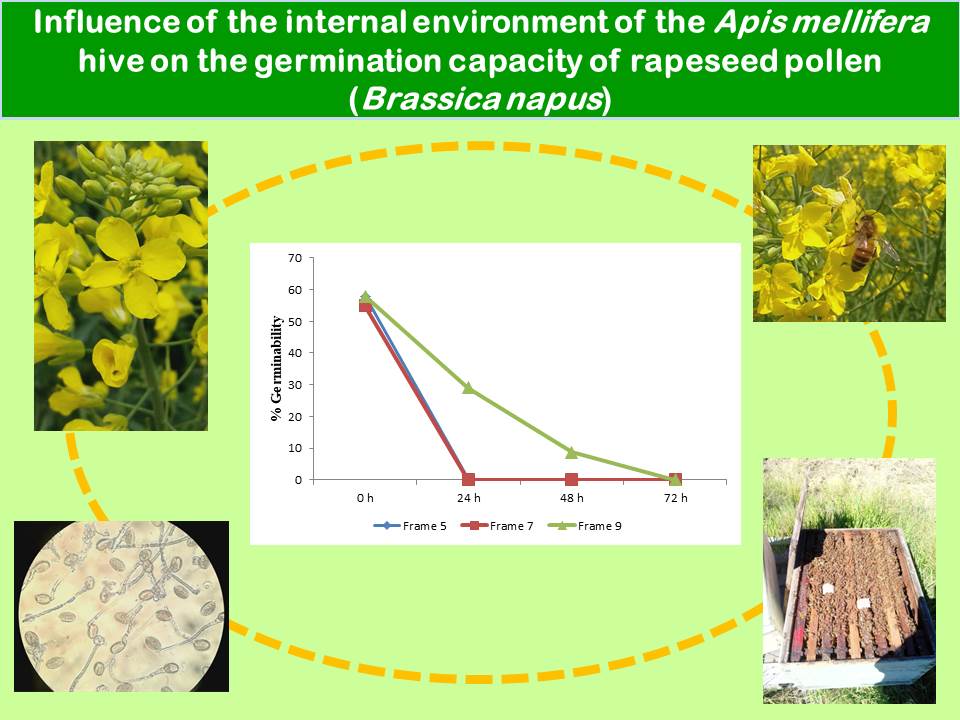
Brassica napus L. (rapeseed, canola) ranks third in worldwide importance among oilseeds. The production of hybrid rapeseed seed requires an androsterile female parent; therefore, fertilization is possible through pollinators carrying viable pollen from an androfertile line. To ensure high pollinator populations, hives are used. However, little is known about the risk of transporting viable pollen into hives. The in vitro germinability of pollen exposed to in-hive conditions was evaluated. Samples of rapeseed pollen obtained from potted plants were placed in four hives of Apis mellifera L. In-hive conditions are unfavorable for rapeseed pollen germinability. Brood areas with the highest temperatures showed no germinated pollen grains within 24 h. Starting at 48 h, germinability decreased significantly, with germinated grains showing atrophied tubes. At 72 h, pollen placed away from brood areas lost germinability.
Highlights:
- Pollen grains in the brood area lost their germination capacity after 24 h of exposure to in-hive conditions, markedly reducing the risk of contaminating new seed production plots.
- In the honey areas, pollen germinability decreased substantially after 48 h of exposure to in-hive conditions and germination capacity was completely lost after 72 h.
- The highest germination percentages were obtained with freshly collected pollen (control treatment).
- Fresh pollen had the thickest and most developed pollen tubes. After 24 h in the hive, pollen grains had thinner, shorter, and convoluted pollen tubes. After 48 h, pollen tubes were atrophied.
Descargas
Citas
Alburaki, M.; Corona, M. 2021. Polyurethane honey bee hives provide better winter insulation than wooden hives. Journal of Apicultural Research. 61(2): 190-196.
Angadi, S. V.; Cutforth, H. W.; Miller, P. R.; McConkey, B. G.; Entz, M. H.; Brandt, S. A.; Volkmar, K. M. 2000. Response of three Brassica species to high temperature stress during reproductive growth. Canadian Journal of Plant Science. 80(4): 693-701. https://doi.org/10.4141/P99-152
Astiz, V. 2012. Biología polínica, fecundación y rendimiento en dos genotipos híbridos de girasol (Helianthus annuus L.) alto oleico. Tesis de Magister. Departamento de Agronomía, Universidad Nacional del Sur, Bahía Blanca, Argentina. 80 p.
Bujok, B.; Kleinhenz, M.; Fuchs, S.; Tautz, J. 2002. Hot spotsinthebeehive. Naturwissenschaften. 89: 299-301. https://doi.org/10.1007/s00114-002-0338-7
Crane, E. 1990. Bees and beekeeping science, Practice and World Resources. Cornell University Press. 640 p.
Delaplane, K. S.; Mayer, D. F. 2000. Crop pollination by bees. CABI. Wallingford, U.K. 344 p.
Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.; Robledo, C. W. 2018. Infostat versión 2018. Grupo Infostat, FCA, Universidad Nacional de Córdoba. Argentina. http:// www.infostat.com.ar
Fahrenholz, L.; Lamprecht, I.; Schricker, B. 1992. Calorimetric investigations of different castes of honey bees, Apis mellifera carnica. Journal of Comparative Physiology B. 162: 119-130. https://doi.org/10.1007/BF00398337
Gil-Lebrero, S.; Navas González, F. J.; Gámiz López, V.; Quiles Latorre, F. J.; Flores Serrano, J. M. 2020. Regulation of microclimatic conditions inside native beehives and its relationship with climate in Southern Spain. Sustainability. 12(16): 6431.
Human, H. 2006. Evaluation of the floral rewards of Aloe greatheadii var. davyana (Asphodelaceae), the most important indigenous South African bee plant (Doctoral dissertation, University of Pretoria).
Hüsken, A.; Dietz-Pfeilstetter, A. 2007. Pollen-mediated intraspecific gene flow from herbicide resistant oilseed rape (Brassica napus L.). Transgenic Research. 16: 557-569.
Kendall, D. A. 1973. The viability and compatibility of pollen on insects visiting apple blossom. The Journal of Applied Ecology. 10(3): 847. https://doi.org/10.2307/2401873
Khatun, S.; Flowers, T. J. 1995. The estimation of pollen viability in rice. Journal of Experimental Botany. 46(1): 151-154. https://doi.org/10.1093/jxb/46.1.151
Koeniger, N. 1978. Das Wärmen der Brut bei der Honigbiene (Apis mellifera L.). Apidologie. 9(4): 305-320.
Li, C.; Yu, M.; Chen, F.; Wang, S. 2010. In vitro maturation and germination of Jatropha curcas microspores. International Journal of Agriculture and Biology. 12(4): 541-546.
Mesquida, J.; Renard, M.; Mesquida, B. 1987. Etude préliminaire sur la germination «in vitro» du pollen de colza (Brassica napus L. var. oleifera Metzger) et sur l’évolution dans le temps de son aptitude à germer. Agronomie. 7(6): 409-416. https://doi.org/10.1051/ agro:19870606
Morrison, M. J. 1993. Heat stress during reproduction in summer rape. Canadian Journal of Botany. 71(2): 303-308. https://doi.org/10.1139/b93-031
Morrison, M. J.; Gutknecht, A.; Chan, J.; Miller, S. S. 2016. Characterising canola pollen germination across a temperature gradient. Crop and Pasture Science. 67(4): 317-322.
Ouvrard, P.; Jacquemart, A. L. 2019. Review of methods to investigate pollinator dependency in oilseed rape (Brassica napus). Field Crops Research. 231: 18-29.
Parker, A. J.; Tran, J. L.; Ison, J. L.; Bai, J. D. K.; Weis, A. E.; Thomson, J. D. 2015. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arthropod-Plant Interactions. 9: 197-203.
Pierre, J.; Renard, M. 2002. La longévité du pollen de colza. Oléagineux, Corps gras, Lipides. 9(1): 11-13.
Read, S. M.; Clarke, A. E.; Bacic, A. 1993. Stimulation of growth of cultured Nicotiana tabacum W 38 pollen tubes by poly (ethylene glycol) and Cu(II) salts. Plant Cell Biology Research Centre, School of Botany, University of Melbourne, Parkville, Victoria, Australia. 177: 1-14.
Robinson, S. V.; Cartar, R. V.; Pernal, S. F.; Waytes, R.; Hoover, S. E. 2023. Bee visitation, pollination service, and crop yield in commodity and hybrid seed canola. Agriculture, Ecosystems & Environment. 347: 108396.
Sato, S.; Peet, M. M.; Thomas, J. F. 2002. Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. Journal of Experimental Botany. 53(371): 1187-1195. https://doi. org/10.1093/jexbot/53.371.1187
Shivanna, K. R.; Cresti, M. 1989. Effects of high humidity and temperature stress on pollen membrane integrity and pollen vigour in Nicotiana tabacum. Sexual Plant Reproduction. 2(3): 137-141.
Shivanna, K. R.; Linskens, H. F.; Cresti, M. 1991. Pollen viability and pollen vigor. Theoretical and Applied Genetics. 81(1): 38-42. https://doi.org/10.1007/BF00226109
Simpson, J. 1961. Nest climate regulation in honey bee colonies: Honey bees control their domestic environment by methods based on their habit of clustering together. Science. 133(3461): 1327-1333.
Stabentheiner, A.; Kovac, H.; Brodschneider, R. 2010. Honeybee colony thermoregulation-Regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE. 5(1): e8967. https://doi.org/10.1371/journal.pone.0008967
Stalidzans, E.; Berzonis, A. 2013. Temperature changes above the upper hive body reveal the annual development periods of honey bee colonies. Computers and Electronics in Agriculture. 90: 1-6.
Timmons, A. M.; O’brien, E. T.; Charters, Y. M.; Dubbels, S. J.; Wilkinson, M. J. 1995. Assessing the risks of wind pollination from fields of genetically modified Brassica napus ssp. oleifera. In The Methodology of Plant Genetic Manipulation: Criteria for Decision Making: Proceedings of the Eucarpia Plant Genetic Manipulation Section Meeting held at Cork, Ireland from September 11 to September 14: 1994 (417-423). Springer Netherlands.
USDA, 2022. United States Department of Agriculture. Accessed in June 2023. https://downloads.usda. library.cornell.edu/usda-esmis/files/tx31qh68h/mc87r456n/f7624t039/oilseeds.pdf
Westcott, L.; Nelson, D. 2001. Canola pollination: an update. Bee World. 82(3): 115-129.
Young, L. W.; Wilen, R. W.; Bonham-Smith, P. C. 2004. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany. 55(396): 485-495. https:// doi.org/10.1093/jxb/erh038
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2018 Revista de la Facultad de Ciencias Agrarias UNCuyo

Esta obra está bajo una licencia internacional Creative Commons Reconocimiento-NoComercial-CompartirIgual 3.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan las Políticas Editoriales.












.jpg)




