ORIGINAL ARTICLE
Effect of protein source on in situ digestibility of sugarcane silage-based diets
Efecto de la fuente de proteína en la digestibilidad in situ de dietas a base de ensilado de caña de azúcar
José Andrés Reyes-Gutiérrez 1, Oziel Dante Montañez-Valdez 1*, Cándido Enrique Guerra-Medina1, 2 Alejandro Ley de Coss 3
1 University of Guadalajara/University Center of the South. Animal Nutrition Research Group. Ave. Enrique Arreola Silva 883. Ciudad Guzmán. 49000. Jalisco. México. * montanez77@hotmail.com
2 National Institute of Forestry, Agriculture and Livestock Research/Rosario Izapa Experimental Campus. Tuxtla Chico. Chiapas. México.
3 Autonomous University of Chiapas. Faculty of Agronomic Sciences. Campus V Villaflores. 30460. Chiapas. México.
Originales: Recepción: 15/02/2018 - Aceptación: 17/12/2018
ABSTRACT
The objective of this study was to evaluate the effect of the protein source in sugarcane silage-based diets on the ruminal pH and in situ dry matter digestibility (DMD). The treatments were: 1)- 60% sugarcane silage + 15% soybean meal (SBM); 2)- 60% sugarcane silage + 15% fish meal (FM); 3)- 55% sugarcane silage + 20% canola meal (CM); and T4)- 50% sugarcane silage + 30% coconut meal (CCM). In situ DMD was determined by the nylon bag technique using four cows equipped with ruminal cannula. Five grams of each experimental diet were weighted in nylon bags and incubated for 8, 12, 24, 48, 72 and 96 h. Dry matter digestibility for SBM, CM, and CCM showed higher values compared to FM. A similar pH among treatments was recorded; however, at 4 h decreases in SBM and FM were observed. Sugarcane silage in integral diets with the different protein sources used in this study, did not modify ruminal pH but showed lower DMD when fish meal was the protein source.
Keywords: Bacterial inoculum; Ruminal kinetic; Tropical forages
RESUMEN
El objetivo de este estudio fue evaluar el efecto de la fuente proteica de dietas a base de ensilado de caña de azúcar sobre el pH ruminal y la digestibilidad in situ de la materia seca (DMD). Los tratamientos fueron: 1)- 60% de ensilado de caña de azúcar + 15% harina de soya (SBM); 2)- 60% ensilado de caña de azúcar + 15% harina de pescado (FM); 3)- 55% ensilado de caña de azúcar + 20% harina de canola (CM); y 4)- 50% de ensilado de caña de azúcar + 30% de harina de coco (CCM). La DMD se determinó mediante la técnica de bolsa de nylon utilizando cuatro vacas equipadas con cánula ruminal. Se pesaron cinco gramos de cada dieta experimental y se incubaron en bolsas de nylon por 8, 12, 24, 48, 72 y 96 h. La digestibilidad de la materia seca para SBM, CM y CCM mostraron los valores más altos en comparación con FM. No hubo cambios en el pH ruminal en los tratamientos, pero, a las 4 h disminuyó en SBM y FM. El ensilado de caña de azúcar en dietas integrales con las diferentes fuentes de proteínas no modifica el pH ruminal, pero reduce la DMD cuando la harina de pescado es la fuente de proteína.
Palabras clave: Inóculo bacteriano; Dinámica ruminal; Forrajes tropicales
INTRODUCTION
In the tropics, grasses are the main source of food for livestock; however, during the drought season, growth and quality of forages is low, affecting animals productivity. Therefore to evaluate alternatives for forage replacement during that period, turns necessary.
Sugarcane is a crop produced in more than 100 countries worldwide, and its biomass production exceeds that of any other forage, making it a good animal feed strategy for sustainable agricultural development in many countries (2).
Sugarcane and particularly sugarcane silage can be an important forage given that it keeps its quality for long periods. However, silage causes losses of up to 30% of dry matter (DM), and concentration of the cell walls components, reducing the in vitro digestibility of DM (6).
Furthermore, silage has high levels of lactic acid and residual carbohydrates, which can potentially inhibit, by pH lowering, microorganisms that spoil the silage, such as yeasts and molds (16). In recent years, there has been increased interest in the use of additives in sugarcane silage, with the objective of inhibiting yeast growth that promote alcoholic fermentation (6). Furthermore, these products have a high protein value and absorbing characteristics that could improve the nutritive value and the fermentation profile by correcting the low protein values of sugarcane and reducing effluent losses. However, strategies have been developed to improve feed intake and reduce sugarcane's nutritional deficiencies by using other ingredients in the ration, allowing sugarcane to be an important fraction of the diet (17).
Studies have demonstrated that diets containing sugarcane and proteic ingredients improve animal performance, take advantage of the high concentration of fermentable carbohydrates, and improve ruminal function (10), but the lack of information on digestibility parameters and ruminal variables of sugarcane silage combined with common protein ingredients has created the need to conduct studies on sugarcane ruminal degradation.
Objective
Provide useful information about the effect of different protein sources on the ruminal digestibility parameters and its effect on rumen pH fluctuations of ensiled sugarcane based diets.
MATERIALS AND METHODS
This study was carried out at Zapotlán El Grande, Jalisco, Mexico, with geographic coordinates of 19°27'13" North latitude and meridians 103°27'57" West longitude, with an altitude of 1,520 m. The biomass of one hectare of sugarcane-variety CP 72-2086, which was approximately 13 months old, second cut-was used in this experiment.
The forage was harvested by hand and chopped in a stationary chopper adjusted for a theoretical cut length of 2.5 cm.
Total biomass was separated into five parts to make the same number of silages. Ensiling was initiated simultaneously in mini silos with 1% bacterial inoculum and 1% additive.
The inoculum consisted of 10.0% molasses, 1.0% commercial yogurt (LALA®; containing: Lactobacillus plantarum, L. bulgaricus, L. casei, L. acidofilus, and L. bifidus), 5.0% chicken manure, 0.5% urea, and 83.0% water; the additive was formulated with 1.0% urea, 0.1% ammonium sulfate, and 0.25% phosphorus.
The silo was opened after 40 days of storage. The treatments were: 1. 60% sugarcane silage + 15% soybean meal (SBM); 2. 60% sugarcane silage + 15% fish meal (FM); 3. 55% sugarcane silage + 20% canola meal (CM); and 4. 50% sugarcane silage + 30% coconut meal (CCM).
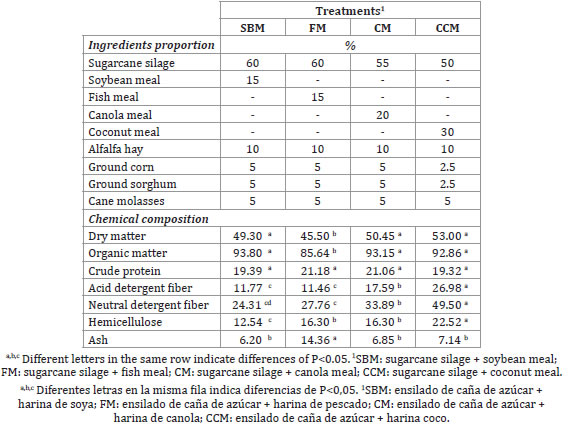
The diets consisted of sugarcane silage with the different protein sources mixed with alfalfa hay, ground corn, ground sorghum, and sugarcane molasses. The Rations were fed in two sessions (AM and PM) to ensure greater cellulolytic activity of rumen microflora. Ad libitum fresh clean water was provided. The experimental diets and analyzed composition of the diets are shown in table 1 (page 347).
Table 1. Ingredients and chemical composition of the experimental diets (%).
Tabla 1. Ingredientes y composición química de las dietas experimentales (%).

Samples of the diets were dried in a circulating air oven at 60°C for 24 h and then milled in a hammer mill equipped with a 2-mm sieve for further analysis.
Total DM was determined using a circulating air oven (100°C for 24 h). Crude protein (CP) was determined by Kjeldahl, ash (A) and organic matter (OM) was calculated by difference using the technique described by the AOAC (2007).
Fiber fractions (NDF and ADF) were determined using alpha amylase without a correction, as specified by Van Soest et al. (1991).
In situ digestibility (DMD) was determined using four 4-year-old Holstein cows (625 ± 63 kg) equipped with permanent rumen cannula with a core diameter of 10 cm (Bar Diamond Lane, Parma, ID, USA). Cows were randomly assigned to a 4 × 4 Latin square and they were housed in individual pens. The statistical model was:
Yijk = μ + Hi + Cj + Tk + εijk
where:
Yijk = the response variable
μ = the general mean
Hi = the effect of the ith period (row)
Cj = the effect of jth animal (column)
Tk = the effect of kth treatment (diet)
εijk = the experimental error
Each period was 15 d, 10 for adaptation to diets and 5 to collect samples. DMD was determined after Vanzant et al. (1998).
Nylon bags were used (10 x 15 cm, pore size 40-60 μm) with 5 g of sample. Each sample was incubated in rumen for 8, 12, 24, 36, 48, 72, and 96 h. Additionally, at each time point, blanks secured with nylon thread to a piece of string (length: 30 cm; weight: 150 g) were added and left suspended in the rumen.
Subsequently, the bags were removed from the rumen according to the incubation times along with the zero hour and washed with running water at low pressure until the water came out just as clear.
Nextly, the bags were dried in a circulating air oven (48 h at 60°C). Ruminal fluid samples were taken from the ruminal cannula at two-h intervals for 12 h, and one was taken one h before daytime feeding (-1, 0, 2, 4, 6, 8, 10 and 12).
Ruminal fluid pH was measured using a portable potentiometer (Model PC18, México) immediately after the rumen fluid was collected. The DMD for the experimental material from each incubation time, was calculated by the weight loss of the samples in bags during ruminal incubation using the model described by Ørskov and McDonald (1979) and modified by McDonald (1981):
P = a + b (1 – e-ct)
where:
a = the washing loss or soluble (%)
b = the insoluble, but potentially digestible fraction (%)
P = the degradation of DM (%)
a + b = potential degradability (%)
c = the fractional degradation rate (h-1)
t = the time (h)
Ruminal turnover constants (k) at 1, 5, and 10 % h-1 were used to model effective degradation (ED;12): ED = a + (b*c) / (c+k). Data from DMD and chemical composition were analyzed using PROC GLM and the ruminal pH with PROC MIXED using the statistical package SAS Version 8.0 (19).
RESULTS AND DISCUSSION
Dry matter and OM content were higher in soybean, canola, and coconut meal, and lower in FM. This last treatment also showed the greatest A concentration.
CP was similar among treatments, but ADF and NDF were higher for CM and CCM.
Hemicellulose concentration was lower in SBM, whereas the greatest concentration was found in CCM. FM and CM resulted similar (table 1, page 347).
Differences were found in DMD (P<0.05) due to the protein supplements of complete diets.
The SBM showed the greatest values while FM had the lowest values. Starting at 12h of incubation, DMD results were more than 50% in all treatments. However, during the following hours, DMD values for FM were the lowest (table 2, page 349).
Table 2. Effect of the protein source on in situ dry matter and ruminal degradability parameters of the experimental diets (%).
Tabla 2. Efecto de la fuente de proteína en la digestibilidad in situ de la materia seca y parámetros de degradabilidad ruminal de las dietas experimentales (%).
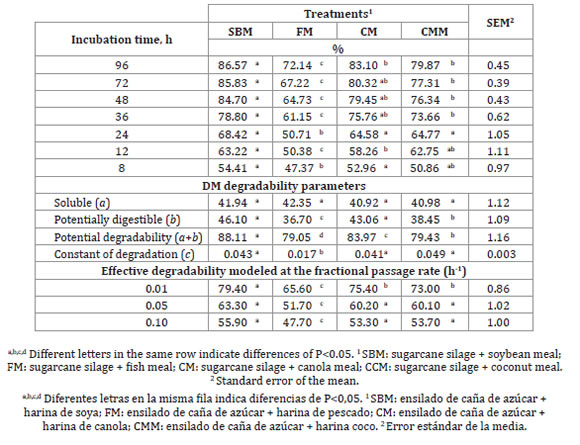
The effective degradability was higher for treatment with SBM, at all times, while FM had the lowest values of the experimental diets (P < 0.05).
Ruminal degradability parameters were similar for soluble fraction of DM (a) across all treatments (P > 0.05). Only FM showed lower values for the rest of the parameters (P < 0.05). Sugarcane silage without additives is characterized by high DM losses (12). Forage-based diets supplemented with protein sources have better amino acid composition and improved nutrient digestibility compared with non-supplemented diets (6, 12).
The improvement in digestibility is due to the greater availability of nutrients required by bacteria for growth and other activities in the rumen. In this study, FM showed the lowest DMD coefficients which is expectable since fish meal has lower rumen degradable protein (RDP) content (3, 4).
However, rumen undegradable protein is necessary to provide essential amino acids to the animal, given that the amount of digested and absorbed protein in the small intestine is an important factor for growth. For this reason, supplementation with rumen undegradable protein provides limiting amino acids, such as lysine and methionine, to the animal.
Fish meal, provided these amino acids in higher concentrations compared with the other protein sources used (9, 15). This difference in by-pass protein might be of importance for producers when adding protein to the diet of growing cattle.
Similar results have been reported by other authors. Van Nhiem et al. (2013) fed Laisind beef cattle with an urea-treated rice straw-based diet supplemented with two different protein sources, FM, and soybean cake and found that diets containing 100% soybean cake had higher DMD compared to FM.
da Silva et al. (2016) compared diets containing corn silage supplemented with SBM and urea and observed an increase in the ruminal digestibility of DM when SBM or urea were added, probably due to the addition of a highly digestible CP source.
The difference among diets of SBM, FM, and CCM in DMD may be explained by the small difference in RDP content. Also, these diets showed higher DM degradability parameters and faster degradation rates, probably due to the higher microbial degradation resulting from a good supply of protein that improved ruminal microbial growth.
The low DMD and ruminal turnover of the FM diets were due to a higher concentration of by-pass protein that may have resulted in limited microbial degradation of nutrients and decreased the efficiency of microbial synthesis in the rumen (14, 21).
Table 3 shows pH values; no differences among treatments were found (P ˃ 0.05).
Table 3. Ruminal pH over time of the experimental diets.
Tabla 3. pH ruminal a través del tiempo de las dietas experimentales.
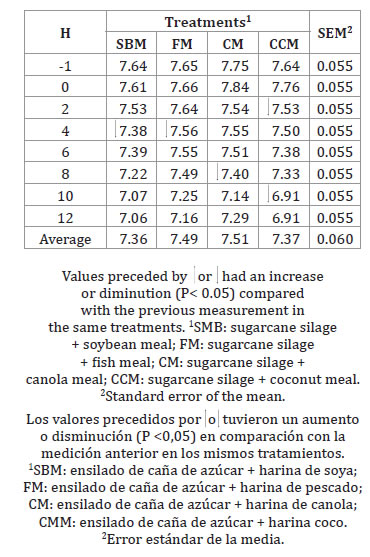
However, differences across sampling time, resulted significant. A reduction was recorded within treatment for SBM and FM at 4 h. Canola meal treatment showed the greatest pH value across incubation time, whereas SBM showed the lowest value. However, all pH values were higher than 7.0, except for CCM at 10 h and 12 h. Russell and Wilson (1996) state that rumen pH may change ruminal cellulose digestion. Low ruminal pH decreased activity, or number of cellulolytic microorganisms, in all experimental diets of their study, while the range of ruminal pH was around the optimum value (6.7-7.0) avoiding reductions in ruminal fermentation. García et al. (2008) and da Silva et al. (2016) reported pH values between 6.62 and 7.2, similar to those found in this experiment.
The high ruminal pH recorded when the experimental diets were fed could be attributed to the natural buffering capacity observed in rations that contain legumes and have high protein concentration (10).
CONCLUSION
The use of sugarcane silage with inoculum and additive in integral diets with the protein sources used in this study did not modify ruminal pH.
However, it reduced the DMD parameters when the protein source was FM, possibly due to its lower content of rumen-degradable protein.
1. AOAC. 2007. Association of Official Analytical Chemist-International. Official Methods of Analysis. 18th ed. AOAC, Arlington. Virginia. USA.
2. Aranda, E. M.; Mendoza, G. D.; Ramos, J. A.; Da Silva, I. C.; Vitti A. C. 2010. Effect of fibrolitic enzymes on rumen microbial degradation of sugarcane fiber. Ciência Animal Brasileira 11: 448-495.
3. Arroquy, J. I.; Cochran, R. C.; Villarreal, M.; Wickersham, T. A.; Llewellyn, D. A.; Titgemeyer, E. C.; Nagaraja, T. G.; Johnson, D. E.; Gnad, D. 2004. Effect of level of rumen degradable protein and type of supplemental non-fiber carbohydrate on intake and digestion of low-quality grass hay by beef cattle. Animal Feed Science and Technology. 115: 83-99.
4. Bohnert, D. W.; Del Curto, T.; Clark, A. A.; Merrill, M. L.; Falck, S. J.; Harmon, D. L. 2011. Protein supplementation of ruminants consuming low-quality cool- or warm-season forage: Differences in intake and digestibility. Journal of Animal Science. 89: 3707-3717.
5. da Silva, L. D.; Pereira, O. G.; Da Silva, T. C.; Valadares Filho, S. C.; Ribeiro, K. G. 2016. Effects of silage crop and dietary crude protein levels on digestibility, ruminal fermentation, nitrogen use efficiency, and performance of finishing beef cattle. Animal Feed Science and Technology. 220: 22-33.
6. Ferreira, D. A.; Gonçalves, L.; Molina, L. R.; Castro-Neto, A.; Tomich, T. R. 2007. Fermentation of sugarcane silage treated with urea, zeolita, bacteria inoculant and bacteria/enzymatic inoculants. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 59: 423-433.
7. García, H.; Abreu, M.; Soto, J. M. 2008. Digestion of residuals of the crop cane treatment with OHNa. 1. Determination of digestibility in situ. Revista Electronica de Veterinaria. 11: 1-8.
8. McDonald, I. 1981. A revised model for estimation of protein degradability in the rumen. Journal of Agricultural Science. 96: 251-252.
9. Mendoza, G. D.; Oviedo, M. F.; Pinos, J. M.; Lee-Rangel, H. A.; Vázquez, A.; Flores, R.; Pérez, F.; Roque, A.; Cifuentes, O. 2020. Milk production in dairy cows supplemented with herbal choline and methionine. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 52(1): 332-343.
10. Molina, A. S.; Sierra, J. F.; Febles, I. 1999. Sugar cane silage with protein synthesis: combined effect of additives and density. Cuban Journal of Agricultural Science. 33: 205-208.
11. Montañez-Valdez, O. D.; Solano-Gama, J. J.; Martínez-Tinajero, J. J.; Guerra-Medina, C. E.; Ley de Coss, A.; Orozco-Hernandez, R. 2013. Buffering capacity of common feedstuffs used in ruminant diets. Revista Colombiana de Ciencias Pecuarias. 26: 37-41.
12. Oliveira, A. C.; Garcia, R.; Pires, A. J. V.; Oliveira, H. C.; Almeida, V. V. S.; Silva, R. R.; Nascimento Filho, C. S.; Abreu Filho, G. 2015. Chemical composition and fermentation characteristics of sugar cane silage enriched with detoxified castor bean meal. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 67: 181-188.
13. Ørskov, E. R.; McDonald, L. 1979. Estimation of protein degradability in the rumen from incubation measurement weighted according to rate of passage. Journal of Agricultural Science. 96: 499-503.
14. Ortiz, M. A.; Ørskov, E. R.; Milne, J.; Galina, H. M. A. 2007. Effect of different sources of nitrogen on in situ degradability and feed intake of Zebu cattle fed sugarcane tops (Saccharum officinarum) Animal Feed Science and Technology. 139: 143-158.
15. Rezai, F.; Zamani, F.; Vatankhah, M. 2012. Effect of rumen undegradable protein (RUP) on colostrum quality and growth of lori Bakhtiari lambs. Global Veterinaria. 8: 93-100.
16. Rocha, K. D.; Pereira, O. G.; Valadares, F. S. C.; Oliveira, A. P.; Pacheco, L. B. B.; Chizzotti, F. H. M. 2006. Valor nutritivo de silagens de milho (Zea mays L.) producidas com inoculantes enzimobacterianos. Revista Brasileira de Zootecnia. 35: 389-395.
17. Rodríguez, D.; Martín, P. C.; Alfonso, F.; Enríquez, A. V.; Sarduy, L. 2009. Performance of crossbred steers (Holstein x Zebu) fed sugarcane forage alone or as part of the diet. Cuban Journal of Agricultural Science. 43: 231-234.
18. Russell, B. J.; Wilson, B. D. 1996. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? Journal of Dairy Science. 79: 1503-1509.
19. SAS. 1999. User's Guide: Statistics. version 8.0. Ed. SAS Institute, Inc., Cary N. C. En CD-ROM.
20. Van Nhiem, D.; Berg, J.; Kjos, N. P.; Trach, N. X.; Tuan, B. Q. 2013. Effects of replacing fish meal with soy cake in a diet based on urea-treated rice straw on performance of growing Laisind beef cattle. Tropical Animal Health and Production. 45: 901-909.
21. Van Soest, P. J. 1994. Nutritional ecology of the ruminant. Cornell University Press.
22. Van Soest, P. J.; Robertson, J. B.; Lewis, B. A. 1991. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. Journal of Dairy Science. 74: 3583-3597.
23. Vanzant, E. S.; Cochran, R. C.; Titgemeyer, E. C. 1998. Standardization of in situ techniques for ruminant feedstuff evaluation. Journal of Animal Science. 76: 2717-2729.