
ORIGINAL ARTICLE
Incidence, prevalence and persistence of bovine venereal diseases in La Pampa (Argentina): estimations for the period 2007 - 2020
Incidencia, prevalencia y persistencia de enfermedades venéreas de los bovinos en La Pampa (Argentina): estimaciones para el período 2007 - 2020
Leonardo L. Molina 1,2, Antón García 3, Elena Angón 3*, Ricardo Moralejo 1, Javier Caballero-Villalobos 3, José Perea 3
1 National University of La Pampa. School of Veterinary Medicine. Calle 5 Esq. 116, 6360. General Pico. La Pampa. Argentina. leonardoluismolina@gmail.com
2 National Service of Health and Agro-Food Quality (SENASA). Centro Regional La Pampa-San Luis. Corrientes 80. Santa Rosa 6300. Argentina.
3 University of Cordoba. School of Veterinary. Department of Animal Production. Crta. Madrid-Cádiz km. 396-a. 14071.Córdoba, Spain. *Corresponding author: E-mail address: eangon@uco.es (E. Angón)
Originales: Recepción: 12/12/2017 - Aceptación: 14/08/2018
ABSTRACT
The venereal diseases Bovine Trichomoniasis (BT) and Bovine Genital Campylobacteriosis (BGC) cause economic losses in endemic areas, such as the province of La Pampa in Argentina, where bovine production is typically extensive. This study used data compiled from 2007 to 2013 by the Official Program for the Control and Eradication (PCE) of venereal diseases, to determine the prevalence, incidence and persistence of BT and BGC and to provide projections up to 2020. Fourteen univariate models were used to adjust each time series. The prevalence and incidence of both diseases significantly decreased during the studied period, while the persistence has remained constant. The prevalence of BT has diminished from 7.48% in 2007 to 3.03% in 2013, while the prevalence of BGC has diminished from 9.36% to 3.15%. The incidences have been reduced to an annual average of 0.60 for BT and 0.67 for BGC. Although the estimation models are not able to accurately predict the future epidemiologic rates of BT and BGC in La Pampa, projections show a significant decreasing trend of the prevalence and incidence of BT and BGC. The persistence of BGC is expected to remain close to the 2007-2013 average, while the persistence of BT did not adjust to any of the 14 models used. These results indicate that PCE has been effective in reducing the infection of disease-free herds. However, in order to reduce the ratio of persistent herds, other preventive and management measures should be considered.
Keywords: Bovine; Bovine genital campylobacteriosis; Monitoring venereal diseases; Bovine trichomoniasis; Modelling
RESUMEN
Las enfermedades venéreas Tricomoniasis bovina (BT) y Campilobacteriosis genital bovina (BGC) causan pérdidas económicas en áreas endémicas, como en la provincia de La Pampa en Argentina, donde la producción bovina es típicamente de carácter extensivo. Este estudio ha utilizado datos compilados de 2007 a 2013 por el Programa Oficial para el Control y la Erradicación (PCE) de enfermedades venéreas, con el objetivo de determinar la prevalencia, incidencia y persistencia de BT y BGC y proporcionar proyecciones hasta 2020. Catorce modelos univariantes fueron utilizados para ajustar cada serie temporal. La prevalencia e incidencia de ambas enfermedades han disminuido significativamente durante el período estudiado, mientras que la persistencia se ha mantenido constante. La prevalencia de BT ha disminuido del 7,48% en 2007 al 3,03% en 2013, mientras que la prevalencia de BGC ha disminuido del 9,36% al 3,15%. Las incidencias se han reducido a un promedio anual de 0,60 para BT y 0,67 para BGC. Aunque los modelos de estimación no pueden predecir con precisión las tasas epidemiológicas futuras de BT y BGC en La Pampa, las proyecciones muestran una tendencia decreciente significativa de la prevalencia e incidencia de BT y BGC. Se espera que la persistencia de BGC se mantenga cerca del promedio de 2007-2013, mientras que la persistencia de BT no se ajustó a ninguno de los 14 modelos utilizados. Estos resultados indican que PCE ha sido eficaz para reducir la infección de rebaños libres de enfermedades. Sin embargo, para reducir la proporción de rebaños persistentes, se deben considerar otras medidas preventivas y de manejo.
Palabras clave: Bovino; Campilobacteriosis genital bovina; Monitoreo de enfermedades venéreas; Tricomoniasis bovina; Modelización
INTRODUCTION
Bovine Trychomoniosis (BT) and Bovine Genital Campylobacteriosis (BGC) are venereal diseases of economic importance, characterized by infertility, embryonic death, abortions, irregular reproductive cycles and long culling intervals (6). BGC infected herds can reduce fertility rates up to 20% and increase abortion rate up to 10% (16). Additionally, sterility may occur in about 11% of infected heifers (24). BT has also been associated to low weights at birth and reductions of more than 50% in the weaning rate (4, 35). In the USA infection produced by BT is estimated to generate economic losses of over 650 million dollars (48).
BT is caused by Tritrichomonas foetus and BGC by Campylobacter fetus veneralis (11, 45). Both diseases are transmitted during coitus, being the bulls asymptomatic carriers. When they reach 3 or 4 years of age they remain as permanently infected reservoirs, while cows are usually recovered after a period of 6-12 months (1, 8, 22). Furthermore, there is neither treatment nor vaccination effective enough for these diseases (5, 7, 52).
Both diseases are distributed worldwide, although they tend to be endemic in areas where bovine production is typically extensive and based on natural breeding, such as the province of La Pampa in Argentina (15, 31).
The economic importance of the bovine sector and the concern for the low reproductive efficiency led to the implementation in 2006 of a Provincial Program for the Control and Eradication (PCE) of BT and BGC. The inclusion in PCE is compulsory for all herds, and positive animals must be removed from the herd within 120 days. However, animals can be medically treated as long as negativity is certified through three post-treatment negative tests (29).
Data generated by PCE provides an opportunity to determine epidemiological indicators for BT and BGC. Furthermore, in Argentina no epidemiological indicators are generated at a national level. In these situations where there is no existing information about occurrence at a national level, the estimations and projections represent and essential tool in order to understand the health requirements and, consequently, to establish prevention and control measures (30, 53). However, aspects such as resistance to diseases, reproductive characteristics or adaptation to difficult environments will always be of value and should be target of greater scientific and informative efforts (34).
Different estimation methods have been used in order to predict occurrence rate, including decomposition methods, ARIMA models, Bayesian models or linear regression (20, 26, 42, 46, 57). The advantages and applicability conditions are specific of each model of analysis and depend on the type of data that constitute the time series (10).
The present study aims to determine population epidemiological indicators for BT and BGC in the province of La Pampa (Argentina) for the period 2007 – 2013, and to provide projections for the incidence, prevalence and persistence of both diseases until 2020.
MATERIAL AND METHODS
Study area and population
The study area was the province of La Pampa in Argentina, which includes approximately 6% of the total bovine population of the country (43). La Pampa is located in the geographic center of Argentina and covers an area of around 143.440 km2 (approximately about 5.2% of Argentina). Farm production in La Pampa is extensive and involves two main production systems: herds that produce calves for fattening establishments and herds where breeding, rearing and fattening are carried out on the same premises (full-cycle herds).
The study population consists in all herds (from 2,000 to 6,000) annually tested under PCE from January, 1st to December, 31rd 2013. PCE regulation in La Pampa requires BT and BGC testing of all nonvirgin bulls in the herd in order to authorize the movement of cattle to another herd, feedlot or slaughterhouses (43). Therefore, the study population corresponds to all the existing herds in La Pampa between 2007 and 2013, except the few herds with no animal movements during this period.
All non-virgin bulls in La Pampa are tested twice a year as part of PCE. The methodology for sample collection and diagnosis is thoroughly described by Molina et al. (29). A bull is classified as negative if the results obtained in two consecutive tests are negative, and positive if at least one test yielded positive results (34). Herds with at least one positive bull were classified as positive.
Data
This study used annual data gathered and reported by PCE from January, 1st 2007 to December, 31rd 2013. Annual prevalence, incidence and persistence of BT and BGC were analyzed.
Prevalence is defined as the ratio of positive herds to the total tested herds. Incidence is defined as the ratio of new positive herds to the total tested herds. Persistence is defined as the ratio of positive herds in the year n that were also positive in the year n – 1 to the total tested herds.
Estimation methods
In order to characterize the behavior of each time series, different models have been built and evaluated using prevalence, incidence and persistence as dependent variables (Y) and time as the independent variable (X). Overall, 14 models have been evaluated for each time series: random walk, random walk with drift, constant mean model, lineal trend model, quadratic trend model, exponential growth trend model, S-curve model, simple exponential smoothing, Brown’s lineal exponential smoothing, Holt’s exponential smoothing, quadratic exponential smoothing, ARIMA (1,0,0) and ARIMA (1,0,1).
Coefficients for each model have been estimated using least square method and contrasted by t-tests (2, 13). Adjustments were determined by the root mean square error (RMSE), the mean absolute error (MAE) and the mean absolute percentage error (MAPE). Adequacy was contrasted using white noise tests to check if the residuals were independent and normally distributed (14). It is possible that several models could be identified for each time series, so it is necessary to choose an optimum model. This optimum model was determined based on Akaike information criterion (AIC) and on Schwartz Bayesian criterion (SBC) (19). All models were retrospectively validated by comparing the means of the obtained estimates and those observed during the period 2007–2013 (45).
All statistical analysis was performed with a significance level of alpha ≤ 0.05 and using the software SPSS v.15.0.
RESULTS
Table 1 shows the prevalence of BT and BGC registered during the period 2007 – 2013 in La Pampa.
Table 1. Herd-level prevalence of BT and BGC during the period 2007–2013 in La Pampa (Argentina).
Tabla 1. Prevalencia a nivel de rebaño de BT y BGC durante el período 2007-2013 en La Pampa (Argentina).

An average 9.51% of the sampled herds showed at least one bull positive to BT or BGC. Herds infected with either of the two diseases have been reduced from 14.18% in 2007 to 5.57% in 2013, involving an annual average decrease of 0.78%. Herds with positive bulls to both diseases averaged 1.60%. Co-infection has been reduced by an annual average of 0.18%, from 2.66% in 2007 to 0.60% in 2013.
Prevalence of BT and BGC has been reduced by an annual average of 0.44% and 0.51%, respectively. Prevalence of BT has decreased from 7.48% in 2007 to 3.03% in 2013, while the prevalence of BGC has diminished from 9.36% to 3.15%.
The average persistence of BT and BGC was 26.78% and 18.98%, respectively. While the persistence of BT has been reduced from 53.03% in 2007 to 39.13% in 2013, the persistence of BGC has slightly increased from 25.93% to 26.47% in 2013 (table 2).
Table 2. Persistence and incidence of BT and BGC during the period 2007-2013 in La Pampa (Argentina).
Tabla 2. Persistencia e incidencia de BT y BGC durante el período 2007-2013 en La Pampa (Argentina).
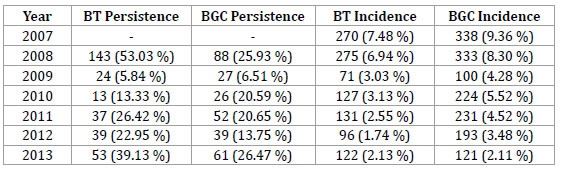
The incidence of both diseases has been reduced by an annual average of 0.61% for BT and 0.67% form BGC.
The projections reveal a reduction of the rates of prevalence and incidence of BT and BGC (table 3).
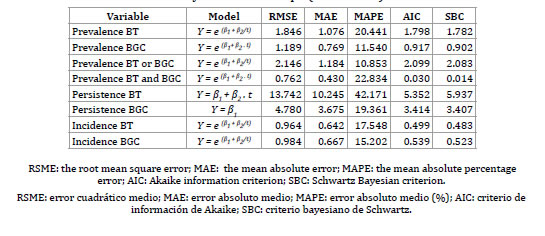
Table 3. Estimation models with the best adjustment for epidemiological indicators of BT and BGC in La Pampa (2007-2013).
Tabla 3. Modelos de estimación con el mejor ajuste para indicadores epidemiológicos de BT y BGC en La Pampa (2007-2013).
Results exposed in table 4 (page 325) indicate that the expected changes are statistically significant.
Table 4. Coefficients of the estimation models of epidemiological indicators of BT and BGC with the best adjustment in La Pampa (2007-2013).
Tabla 4. Coeficientes de los modelos de estimación de indicadores epidemiológicos de BT y BGC con el mejor ajuste en La Pampa (2007-2013).
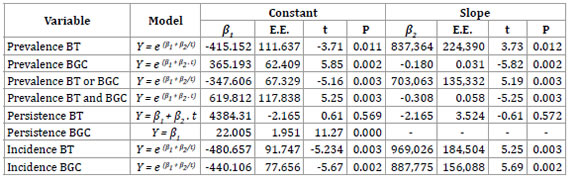
The prevalence of BT showed the best adjustment with the S-curve model. An annual reduction of BT prevalence by 0.25% is expected, reaching 0.54 % (0.10–2.07 95% CI) in 2020.
The prevalence of BGC was adjusted with the exponential growth trend model and shows an expected annual decrease of 0.35%, reaching 0.94% (0.38–1.93 95% CI) in 2020.
The prevalence of BT and BGC was adjusted with the S-curve model. Herds infected by either one disease are expected to be reduced by an annual average of 0.52%, reaching 1.56% (0.58-3.18 95% CI) in in 2020 (figure 1, page 326).

Figure 1. Adjustment and prediction of the prevalence of BT or BGC in La Pampa with the S-curve model.
Figura 1. Ajuste y predicción de la prevalencia de BT o BGC en La Pampa con el modelo de curva en S.
Co-infection by BT and BGC was adjusted with the exponential growth trend model and shows an expected annual decrease of 0.06% (figure 2, page 326).
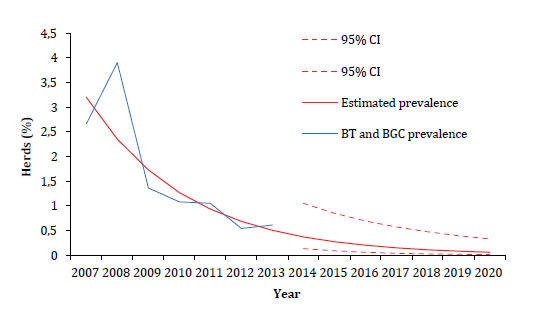
Figure 2. Adjustment and prediction of the prevalence of BT and BGC in La Pampa with the exponential growth trend model.
Figura 2. Ajuste y predicción de la prevalencia de BT y BGC en La Pampa con el modelo de tendencia de crecimiento exponencial.
The incidences of BT and BGC were adjusted with the S-curve model and showed and expected annual decrease of 0.19% and 0.28%, respectively.
The persistence of BT was adjusted with the lineal trend model, although it was not statistically significant (table 4). The best adjustment for persistence of BGC was with the constant mean model, what suggests that persistence will remain constant at about 22% during the period 2013-2020.
DISCUSSION
The accumulated data in La Pampa during 7 years have been used to determine the population epidemiological indicators of BT and BGC in the province. The initial prevalence of both diseases was generally lower than those reported in other endemic areas in Asia, Australia, North America, South America and South Africa (3, 4, 12, 21, 22, 24, 33, 41, 56). A prevalence of 37% for BGC was reported in Uruguay, while no herds were found to be positive to BT (39). In Buenos Aires (Argentina) a prevalence of 1.5% was reported for BGC and of 19.4% for BT (40). In the east of La Pampa, a prevalence of 11.1% was found for BT and of 7.0% for BGC (49). Most of the reported data of prevalence comes from studies conducted with few herds and limited conditions, so it is possible that they do not accurately reflect the actual situation. For instance, in South Africa, the occurrence of BGC seems to be vastly underestimated (31).
The detection of BT and BGC in La Pampa has been based on the collection of two consecutive preputial smegma samples and on cultural methods (29).
There are, however, different factors that could negatively affect the accuracy of the diagnoses, being some of them easily upgradable. For instance, sample collection is not always effective due to the intermittent presence of pathogenic agents in the foreskin of bulls (17, 27). Furthermore, the frequency and the sampling intervals affect the effectiveness of the diagnosis techniques (32, 37). Although none of them is 100% sensible, PCR based techniques represent an improvement compared to those obtained by bacterial culture (25, 50). According to Yao et al. (2013), an unpredictable and often ignored factor is the delay in the delivery of samples to the diagnostic laboratories after sample collection. Another factor is the existence of positive cows that are currently not detected as they are not included in PCE. In order to improve accuracy in diagnosis, it would be highly recommended to consider these factors and to analyze the relationship between the sample collection and the situation of the herd.
Prevalence of BT and BGC reached their highest peak in 2008. Ever since, they have decreased, proving effective control measures. In Wyoming (USA), a similar to PCE control plan, managed to reduce the herd-level prevalence of BT to 1.29% in nine years (55). Besides, the estimation models show a decreasing trend, and both prevalences are expected to continue diminishing in the future, although the models are not capable of accurately predicting the levels of prevalence. This is partly due to the fact that only data from 2007 to 2013 is available. The decrease of the prevalence of BT and BGC is mainly due to a reduction of new infections, although the proportion of persistent herds has remained broadly stable. Although they cannot precisely predict the levels of incidence and persistence, estimation models suggest that in the future, incidence will continue to decrease while persistence will remain constant.
PCE has been especially useful to reduce the infection of disease-free herds. This is explained by the reposition with bulls certified as negative to both diseases. However, PCE has not managed to significantly reduce the rate of persistent herds. On one side, the disposal of positive bulls might not be enough to eliminate the disease from the flock, resulting also necessary to remove breeding cows or to establish a period of reproductive rest during 6-12 months to facilitate recovery (22, 38). On the other hand, it could be possible to eliminate the disease replacing positive bulls, although effective measures to prevent new infections are not simultaneously taken. In this sense, in the areas of higher BT and BGC risk in La Pampa, the exchange of bulls between farmers and pasture sharing, are very common (29).
According to Yao et al. (2013), in order to eradicate the disease, it is not enough to detect and remove the positive bulls, but also replacing them with disease-free bulls. In addition to PCE, other preventive and herd management measures should be considered. BT and BGC share in La Pampa some of their main risk factors (15, 18, 23, 27). Besides, there is some spatial correlation between the risk of BT and BGC (29). Thus, the development of integrated actions focused on the common features of BT and BGC should enhance the effectiveness and efficiency of the intervention methods (9).
Taking into consideration the common features of both diseases and the productive conditions in La Pampa, the priority actions should focus on the improvement of the reproductive control and keeping the herd in a closed cycle. Practices as seasonal breeding or rectal examination are not usual in the province of La Pampa and yet could greatly improve reproductive efficiency and control of venereal diseases. On one side, they allow the early identification of reproductive failures and the discard of non-breeding cows. On the other hand, testing bulls before breeding and after a period of sexual repose, reduces the probability of false negatives due to low concentration of microorganisms, and avoids positive bulls to encounter other cows in the herd (28). Finally, keep the herd in a closed cycle prevents contact with animals from other herds with an unknown sanitary status. In this sense, it is important to keep wiring and perimeter fences in good conditions, avoiding the exchange of bulls and the sharing of pastures (18, 23). According to Yao et al. (2013) eradication of BT and BGC is only possible through the replacement of natural breeding by artificial insemination. However, although artificial insemination generally involves a considerable reduction of the rates of occurrence or even eradication, there are typically extensive areas free of BT and BGC where artificial insemination is not in practice, such as the in Spanish grasslands (27, 51).
CONCLUSIONS
Univariate analysis was an effective tool for modeling the historical and future prevalence, incidence and persistence of T. foetus and C. fetus infections in La Pampa (Argentina). Prevalence and incidence of both diseases have significantly decreased during the studied period, while the persistence has remained constant. The estimation models show projections with a significant decreasing trend of the prevalence and incidence of BT and BGC, indicating that PCE has been effective to reduce the infection of disease-free herds. However, in order to reduce the ratio of persistent herds, other preventive and management measures should be considered.
1. Anderson, M. L. 2007. Infectious causes of bovine abortion during mid-to late-gestation. Theriogenology. 68: 474-486.
2. Angón, E.; García A.¸ Perea, J.; Acero, R.; Toro-Mújica, P.; Pacheco, H.; González, A. 2013. Eficiencia técnica y viabilidad de los sistemas de pastoreo de vacunos de leche en la Pampa. Argentina. Agrociencia. 47: 443-456.
3. Bawa, E. K.; Adekeye, J. O.; Oyedipe, E. O.; Umoh, J. U. 1991. Prevalence of bovine campylobacteriosis in indigenous cattle of three states in Nigeria. Tropical Animal Health and Production. 23:157-160.
4. BonDurant, R. H.; Anderson, M. L.; Blanchard, P.; Hird, D.; Danaye-Elmi, C.; Palmer, C.; Sischo, W. M.; Suther, D.; Utterback, W.; Weigler, B. J. 1990. Prevalence of trichomoniasis among California beef herds. Journal of the American Veterinary Medical Association. 10: 1590-1593.
5. BonDurant, R. H. 2005. Venereal diseases of cattle: natural history, diagnosis, and the role of vaccines in their control. Veterinary Clinics of North America: Food Animal Practice. 21: 383-408.
6. Campero, C. M.; Rodriguez Dubra, C.; Bolondi, A.; Cacciato, C.; Cobo, E.; Perez, S.; Odeon, A.; Cipolla, A.; BonDurant, R. H. 2003. Two-step (culture and PCR) diagnostic approach for differentiation of non-T. foetus trichomonads from genitalia of virgin beef bulls in Argentina. Veterinary Parasitology. 112: 167-175.
7. Cobo, E. R.; Morsella, C.; Cano, D.; Cipolla, A.; Campero, C. M. 2004. Immunization in heifers with dual vaccines containing Tritrichomonas foetus and Campylobacter fetus antigens using systemic and mucosal routes. Theriogenology. 62: 1367-1382.
8. Corbeil, L. B.; Campero, C. M.; Rhyan, J. C.; BonDurant, R. H. 2003. Vaccines against sexually transmitted diseases. Reproductive Biology and Endocrinology. 1: 118.
9. Cowie, C. E.; Marreos, N.; Gortázar, C.; Jaroso, R.; White, P. C. L.; Balseiro, A. 2014. Shared risk factors for multiple livestock diseases: A case study of bovine tuberculosis and brucellosis. Research in Veterinary Science. 97: 491-497.
10. Diggle, P. 1990. Time Series: A Biostatistical Introduction. Oxford University Press. Oxford. UK.
11. Eaglesome, M. D.; Garcia, M. M. 1992. Microbial agents associated with bovine genital tract infections and semen. Part 1. Brucella abortus, Leptospira, Campylobacter fetus and Tritrichomonas foetus. Veterinary Bulletin. 62: 743-775.
12. Erasmus, J. A.; De Wet, J. A. L.; Van der Merwe, H. E.; Pienaar, G. C. J. 1989. Bovine trichomoniasis in the north western Cape Province, western Transvaal and the Orange Free State. Journal of the South African Veterinary Association. 60: 51-52.
13. Faruk, D. Ö. 2010. A hybrid neural network and ARIMA model for water quality time series prediction. Engineering Applications of Artificial Intelligence. 23(4): 586-94.
14. Fong, P. W.; Li, W. K. 2003. On time series with randomized unit root and randomized seasonal unit root. Computational statistics & data analysis. 43(3): 369-95.
15. Gay, J. M.; Ebel, E. D.; Kearley, W. P. 1996. Commingled grazing as a risk factor for trichomonosis in beef herds. Journal of the American Veterinary Medical Association. 209: 643-646.
16. Hum, S. 1987. Bovine abortion due to Campylobacter fetus. Australian Veterinary Journal. 64: 319-320.
17. Irons, P. C.; Henton, M. M.; Bertschinger, H. J. 2002. Collection of preputial material by scraping and aspiration for the diagnosis of Tritrichomonas foetus in bulls. The Journal of the South African Veterinary Association. 73: 66–69.
18. Jiménez, D. F.; Perez, A. M.; Carpenter, T. E.; Martinez, A. 2011. Factors associated with infection by Campylobacter fetus in beef herds in the Province of Buenos Aires, Argentina. Preventive Veterinary Medicine. 101: 157-162.
19. Koehler, A. B.; Murphree, E. S. 1988. A comparison of the Akaike and Schwarz criteria for selecting model order. Applied Statistics. 37: 187-95.
20. Luz, P. M.; Mendes, B. V. M.; Codeao, C. T.; Struchiner, C. J.; Galvani, A. P. 2008. Time series analysis of dengue incidence in Rio de Janeiro, Brazil. American Journal of Tropical Medicine Hygiene. 79: 933-939.
21. Mai, H. M.; Irons, P. C.; Kabir, J.; Thompson, P. N. 2013. Prevalence of bovine genital campylobacteriosis and trichomonosis of bulls in northern Nigeria. Acta Veterinaria Scandinavica. 55: 56.
22. Mancebo, O. A.; Russo, A. M.; Carabajal, L. L.; Monzon, C. M. 1995. Persistence of Tritrichomonas foetus in naturally infected cows and heifers in Argentina. Veterinary Parasitology. 59: 7-11.
23. Mardones, F. O.; Perez, A. M.; Martínez, A.; Carpenter, T. E. 2008. Risk factors associated with Tritrichomonas foetus infection in beef herds in the Province of Buenos Aires. Argentine. Vet. Parasitol. 153: 231-237.
24. McCool, C. J; Townsend, M. P.; Wolfe, S. G.; Simpson, M. A.; Olm, T. C.; Jayawardhana, G. A.; Carney, J. V. 1988. Prevalence of bovine venereal disease in the Victoria River district of the northern territory: Likely economic effects and practical control measures. Australian Veterinay Journal. 65: 153-156.
25. McMillen, L.; Lew, A. E. 2006. Improved detection of Tritrichomonas foetus in bovine diagnostic specimens using a novel probe-based real time PCR assay. Veterinary Parasitology. 141: 204-215.
26. Medina, D. C.; Findley, S. E.; Doumbia, S. 2008. State–space forecasting of Schistosoma haematobium time-series in Niono, Mali’, PLoS Neglected Tropical Diseases. 2: 1-12.
27. Mendoza Ibarra, J. A.; Pedraza Díaz, S.; García Peña, F. J.; Rojo Montejo, S.; Ruíz Santa Quiteria, J. A.; San Miguel Ibañez, E.; Navarro Lozano, V.; Ortega Mora, L. M.; Osoro, K.; Collantes Fernandez, E. 2011. High prevalence of Tritrichomonas foetus infection in Asturiana de la Montaña beef cattle kept in extensive conditions in Northern Spain. The Veterinary Journal. 193(1): 146-51.
28. Michi, A. N.; Favetto, P. H.; Kastelic, J.; Cobo, E. R. 2016. A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health. Theriogenology. 85: 781-791.
29. Molina, L.; Perea, J.; Meglia, G.; Angon, E.; Garcia, A. 2013. Spatial and temporal epidemiology of bovine trichomoniasis and bovine genital campylobacteriosis in La Pampa province (Argentina). Preventive Veterinary Medicine. 110: 388-94.
30. Moller, B.; Fekjaer, H.; Hakulinen, T.; Tryggvadottir, L.; Storm, H. H.; Talback, M.; Haldoresen, T. 2002. Prediction of cancer incidence in the Nordic countries up to the year 2020. European Journal of Cancer Prevention. 11: Suppl 1: S1-96.
31. Mshelia, G. D.; Amin, J. D.; Woldehiwet, Z.; Murray, R. D.; Egwu, G. O. 2010. Epidemiology of bovine venereal campylobacteriosis: Geographic distribution and recent advances in molecular diagnostic techniques. Reproduction in Domestic Animals. 45: 221-230.
32. Mukhufhi, N.; Irons, P. C.; Michel, A.; Peta, F. 2003. Evaluation of a PCR test for the diagnosis of Tritrichomonas foetus infection in bulls: effects of sample collection method, storage and transport medium on the test. Theriogenology. 60: 1269-1278.
33. Pefanis, S. M.; Herr, S.; Venter, C. G.; Kruger, L. P.; Queiroga, C. C.; Amaral, L. 1988. Trichomoniasis and campylobacteriosis in bulls in the Republic of Transkei. Journal of the South African Veterinary Association 59: 139-140.
34. Perea, J.; Barba, C.; Luque, M.; González, A.; Angón, E.; García, A. 2018. Conocimiento científico y políticas de conservación: interrelaciones en las razas ganaderas autóctonas españolas en peligro de extinción. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 50(1): 171-184.
35. Perez, A.; Cobo, E.; Martínez, A.; Campero, C.; Späth, E. 2006. Bayesian estimation of Tritrichomonas foetus diagnostic test sensitivity and specificity in range beef bulls. Veterinary Parasitology. 142: 159-162.
36. Rae, D. O. 1989. Impact of trichomoniasis on the cow-calf producer’s profitability. Journal of the American Veterinary Medical Association. 194: 771-775.
37. Rae, D. O.; Chenoweth, P. J.; Genho, P. C.; McIntosh, A. D.; Crosby, C. E.; Moore, S. A. 1999. Prevalence of Tritrichomonas fetus in a bull population and effect on production in a large cow-calf enterprise. Journal of the American Veterinary Medical Association. 214: 1051-1055.
38. Rae, D. O.; Crews, J. E.; Greiner, E. C.; Donovan, G. A. 2004. Epidemiology of Tritrichomonas foetus in beef bull populations in Florida. Theriogenology 61: 605-618.
39. Repiso, M. V.; Gil, A.; Bañales, P.; D’Anatro, N.; Fernández, L.; Guarino, H.; Herrera, B.; Núñez, A.; Olivera, M.; Osawa, T.; Silva, M. 2005. Prevalencia de las principales enfermedades infecciosas que afectan el comportamiento reproductivo en la ganadería de carne y caracterización de los establecimientos de cría del Uruguay. Veterinaria. 40(157): 5-28.
40. Rojas, M.; Vázquez, P.; Verdier, M.; Campero, C. 2011. Evolución y distribución de las enfermedades de transmisión sexual en bovinos del partido de Rauch, prov. Buenos Aires, años 2001-2009. Revista Veterinaria Argentina. 27(273): 1-14.
41. Ryley, D. E.; Wagner B.; Polley, L. T.; Krieger, J. N. 1995. PCR-Based study of conserved and variable DNA sequences of Tritrichomonas foetus isolated from Saskatchewan, Canada. Journal of Clinical Microbiology. 33: 1308-1313.
42. Sebastiani, P.; Mandl, K. D.; Szolovits, P.; Kohane, I. S.; Ramoni, M. F. A. 2006. Bayesian dynamic model for influenza surveillance’. Statistics in Medicine. 25: 1803-1825.
43. SENASA. Res. 358/2008. Se reconoce el “Programa de control y erradicación de las enfermedades venéreas en bovinos de la Provincia de La Pampa” [on line]. Available from: http://www.senasa.gov.ar/contenido.php?to=n&in=1334&ino=1334&io=7938 (Accessed 12.07.17).
44. SENASA, 2017. Indicadores ganaderos. Existencias bovinas por categoría y departamento 2017. http://www.senasa.gob.ar/cadena-animal/bovinos-y-bubalinos/informacion/ informes-y-estadisticas (Accessed 13/07/2017).
45. Skirrow, S. Z.; BonDurant, R. H. 1988. Bovine trichomoniasis. Veterinary Bulletin. 58: 591-603.
46. Soebiyanto, R. P.; Adimi, F.; Kiang, R. K. 2010. Modeling and predicting seasonal influenza transmission in warm regions using climatological parameters. PLoS One 5, e9450.
47. Souza, D. L. B.; Bernal, M. M. 2012. Incidencia, prevalencia y mortalidad del cáncer renal en España: estimaciones y proyecciones para el periodo 1998-2022. Actas Urológicas Españolas. 36(9): 521-526.
48. Speer, C. A.; White, M. W. 1991. Bovine trichomoniasis. Large Animal Veterinary. 46: 18-20.
49. Suárez, V. H.; Miranda, A. O.; Arenas, S. M.; Schmidt, E. E.; Lambert, J.; Schieda, A.; Felice, G.; Imas, D.; Sola, E.; Pepa, H.; Bugnone, V.; Calandri, H.; Lordi, L. V. 2008. Prevalencia de patologías e incidencia de la sanidad en los sistemas bovinos en el este de la provincia de La Pampa, Argentina. Revista Veterinaria Argentina. 25: 258-280.
50. Szonyi, B.; Srinath, I.; Schwartz, A.; Clavijo, A.; Ivanek, R. 2012. Spatio temporal epidemiology of Tritrichomonas foetus infection in Texas bulls based on state-wide diagnostic laboratory data. Veterinary Parasitology. 186: 450–455.
51. Taylor, M. A.; Marshall, R. N.; Stack, M. 1994. Morphological differentiation of Tritrichomonas foetus from other protozoa of the bovine reproductive tract. British Veterinary Journal. 150: 73-80.
52. Villarroel, A.; Carpenter, T. E.; BonDurant, R. H. 2004. Development of a simulation model to evaluate the effect of vaccination against Tritrichomonas foetus on reproductive efficiency in beef herds. American Journal of Veterinary Research. 65: 770-775.
53. Wangdi, K.; Singhasivanon, P.; Silawan, T.; Lawpoolsri, S.; White, N. J.; Kaewkungwal, J. 2010. Development of temporal modelling for forecasting and prediction of malaria infections using time-series and ARIMAX analyses: a case study in endemic districts of Bhutan. Malaria Journal. 9: 251.
54. Yao, C. 2013. Diagnosis of Tritrichomonas foetus-infected bulls, an ultimate approach to eradicate bovine trichomoniasis in US cattle? Journal of Medical Microbiology. 62: 1-9.
55. Yao, C.; Bardsley, K. D.; Litzman, E. A.; Hall, M. L.; Davidson, M. R. 2011. Tritrichomonas foetus infection in beef bull populations in Wyoming. Journal of Bacteriology and Parasitology. 2: 117.
56. Yang, N.; Cui, X.; Qian, W.; Yu, S.; Liu, Q. 2012. Survey of nine abortifacient infectious agents in aborted bovine fetuses from dairy farms in Beijing, China, by PCR. Acta Veterinaria Hungarica. 60: 83-92.
57. Zhang, X.; Zhang, T.; Pei, J.; Liu, Y.; Li, X.; Medrano-Gracia, P. 2016. Time Series Modelling of Syphilis Incidence in China from 2005 to 2012. PLoS ONE 11(2): e0149401. doi:10.1371/journal.pone.0149401.