ORIGINAL ARTICLE
Fungal diversity and Fusarium oxysporum pathogenicity associated with coffee corky-root disease in Mexico
Diversidad de hongos y patogenicidad de Fusarium oxysporum asociados a la corchosis de la raíz del cafeto en México
Daniel López-Lima 1, Gloria Carrión 1*, Petra Sánchez-Nava 2, Damaris Desgarennes 1, Luc Villain 3
1 Instituto de Ecología, A.C.-Cluster Biomimic. Carretera antigua a Coatepec 351. Xalapa 91070. Veracruz. México. * gloria.carrion@inecol.mx
2 Universidad Autónoma del Estado de México. Instituto Literario 100. Toluca 50000. Estado de México. Mexico.
3 Cirad. UMR RPB, F- 34394 Montpellier. France.
Originales: Recepción: 18/10/2017 - Aceptación: 01/06/2018
ABSTRACT
The disease known as coffee corky-roots associated to the infection by the root-knot nematode Meloidogyne paranaensis is an important issue for coffee crop in several countries. In Mexico, particularly in the Veracruz state, considerable loses are recorded annually in Coffea arabica plantations by corky-root disease. Previous studies have revealed the presence of fungi in coffee corky-root tissues. However, these fungi have not been yet identified. This work aimed to identify at species level the fungi associated to the coffee corky-root symptoms and determine their pathogenicity on coffee plants. Coffee roots with corky-root symptoms were collected in eight sites distributed through the major coffee growing region of Veracruz. Observations of inside cortical root tissues under scanning electron microscope revealed abundant mycelium and conidia in corky-root samples in contrast with absence of any fungi development in healthy roots. Forty-nine fungi strains from internal corky-root tissue were isolated and identified at species level by ITS sequences. Fusarium oxysporum was the most frequent species and the only present in all of the corky-root samples. These strains were selected for the pathogenicity test. All F. oxysporum strains colonized the vascular system of coffee plants although none caused wilting symptoms.
Keywords: Coffea arabica; Root-knot nematodes; Filamentous fungi
RESUMEN
La corchosis de la raíz del café asociada a la infección del nematodo agallador de la raíz Meloidogyne paranaensis es un importante problema para el cultivo de café en varios países. En México, particularmente en el estado de Veracruz, se registran considerables pérdidas anuales en las plantaciones de Coffea arabica por esta enfermedad. Estudios anteriores han revelado la presencia de hongos en los tejidos afectados con corchosis de la raíz del café. Sin embargo, estos hongos aún no han sido identificados. El objetivo de este trabajo fue identificar a nivel de especie los hongos asociados a la corchosis de la raíz y determinar su patogenicidad en plantas de café. Se recolectaron raíces de cafetos con síntomas de corchosis en ocho sitios distribuidos a través de la principal región cafetalera de Veracruz. Las observaciones de los tejidos internos de las raíces bajo el microscopio electrónico de barrido revelaron abundante micelio y conidios en muestras de raíz con corchosis, en contraste con su ausencia en raíces sanas. Se aislaron 49 hongos de los tejidos internos afectados con corchosis y se identificaron a nivel de especie mediante secuencias de ITS. Fusarium oxysporum fue la especie más frecuente y la única presente en todos los sitios de muestreo, por lo que estas cepas fueron seleccionadas para la prueba de patogenicidad. Todas las cepas de F. oxysporum fueron capaces de colonizar el sistema vascular de las plantas de café, aunque ninguna causó síntomas de marchitez.
Palabras claves: Coffea arabica; Nematodo agallador; Hongos filamentosos
INTRODUCTION
Coffee takes the second place among the most worldwide traded products (after oil) providing economic livelihood to more than 125 million people. During the coffee cycle 2015/2016 the producing countries all together, exported more than 110 million, 60-kg bags (22).
In Mexico, Arabica coffee plantations represent almost the 90% of the coffee production and still plays an important socio economic role in many rural areas with high level of poverty. Additionally, Arabica plantations provide many important ecosystemic services to the country, since this crop is predominantly grown in agroforestry systems and in ecologically sensible mountainous areas (20). However, Mexico coffee exportable production has been decreasing, almost constantly, since the end of the 90’s falling from around 4 million to less than half million of 60-kg bags for the last harvest, 2015/2016 (22). This dramatic decrease in production is caused by different reasons like the aging of most coffee plantations and biotic stresses. In this sense, the coffee leaf rust has affected Mexican coffee crop mainly during the last three years, in addition, to plant-parasitic nematodes which have wide distribution in all coffee growing regions of Mexico (21). Today, coffee leaf rust and plant-parasitic nematodes are the two major phytosanitary problems affecting Arabica coffee plantations throughout Latin America (1, 42).
Nonetheless, while coffee leaf rust incidence and damage are determined by many factors such as, micro and macroclimate conditions and agronomic practices, plant-parasitic nematodes represent a continued and underlying threat for both Arabica and Robusta plantations with a high potential damage. Due to the lack of analysis and detection of plant-parasitic nematodes in nurseries and the fact that seedlings show symptoms after high nematode densities are reached, field nematode infestations continue to expand.
Moreover plant-parasitic nematodes create a continued stress during the entire lifetime of the plantation. No complete eradication by control methods is possible, in addition to the unsafe usage of susceptible germplasm (41). The major nematode damages in Latin America are caused by root-knot nematodes (RKN), Meloidogyne spp. (42), particularly by two species associated with a devastating syndrome called coffee corky-root disease: Meloidogyne arabicida, to date only detected in Costa Rica (24) and M. paranaensis, with a wider distribution, in Brazil (11), Guatemala (42), and Hawaii (11).
In Mexico, coffee corky-root disease has been detected since the 1960s in the state of Veracruz (the second national coffee producing state) and M. paranaensis has been confirmed by using specific SCAR molecular markers as the RKN species linked to coffee corky-root symptoms (25).
The affected coffee trees show a progressive decline, starting with chlorosis followed by flower, leaf and fruit fall, until the death of plants. This occurs in a period between two to four years depending on agro-ecological conditions and mainly when plants begin to produce (5).
The root system of infested plants shows numerous small elongated galls on young white roots and large swelling on older and more lignified roots accompanied by large, deep and cracked cortical tissues, reminding of cork aspect (5). These corky symptoms can affect the primary roots including the taproot, up to the plant crown and even reaching the first centimeters of the stem as observed in this work. Cuttings of these corky root swellings reveal numerous M. paranaensis females with their egg masses (25) It is noteworthy that, in Mexico, the fungus Fusarium oxysporum has been strongly associated to coffee plants with corky-root symptoms (16) along with other fungi like Cylindrocladium sp., Fusarium solani, Trichoderma sp. and Verticillium sp. (36).
After the aforementioned to research those fungi directly associated with internal tissues of coffee roots damaged by nematodes, collected from different sites of coffee regions, tuns necessary. Therefore, the objectives of this work were to: i) observe the presence of fungi in the affected tissues, ii) isolate and identify the fungal community associated with the coffee corky-root disease using molecular methods and iii) conduct pathogenicity test of isolated fungi on coffee plants without the presence of the nematode M. paranaensis.
MATERIALS AND METHODS
Sampling
The sampling of coffee corky-roots was done on eight coffee plantations distributed in the main coffee cropping area of the Veracruz state, located between the eastern slope of the Mexican Trans Volcanic Belt and the southern slope of the Sierra Madre Oriental.
The sampled coffee plantations were selected based on field technical information and on previous studies that registered the presence of the corky-root disease or spots in coffee plantations with affected roots and aerial symptoms such as, chlorosis, deficient growth, defoliation and premature death of plants. On each plantation, roots were taken from 8-9 coffee plants with corky-root symptoms to form one composite sample of each sampling site. In a previous study, it was determined that in all coffee corky-root collected samples the only present RKN was M. paranaensis (25).
Scanning electron microscope observations
For the scanning electron microscope (SEM) observations, 3 months old seedlings of an in vitro propagated F1 intraspecific hybrid line of Coffea arabica (7) cultivated in 6 litres pots filled with previously sterilized substrate were infested with a population of M. paranaensis reared on tomato plants in greenhouse. This population was initially collected on coffee at one of the eight sampling sites of this study, Jilotepec site (table 1, page 281-282).
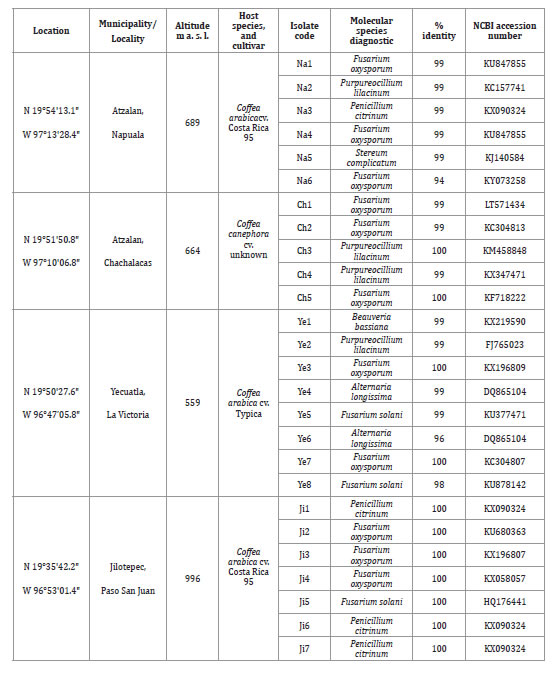
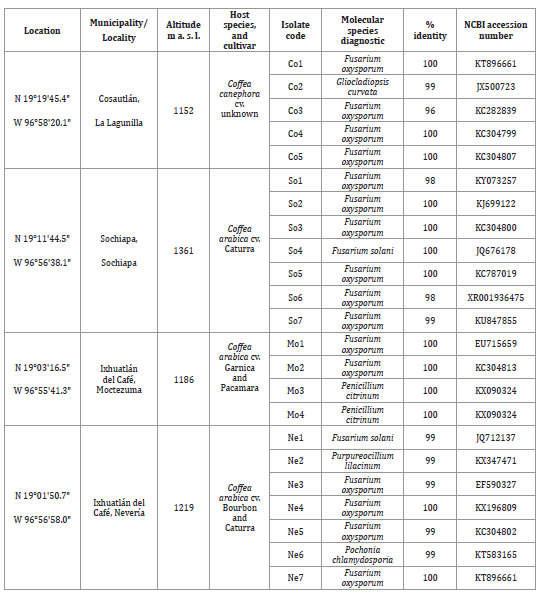
Table 1. Geographic data of sampling sites and molecular identification of fungal species associated with coffee corky-root disease.
Tabla 1. Datos geográficos de los sitios de muestreo e identificación molecular de las especies de hongos asociadas a la corchosis de la raíz del cafeto.

Table 1 (cont.). Geographic data of sampling sites and molecular identification of fungal species associated with coffee corky-root disease.
Tabla 1 (cont.). Datos geográficos de los sitios de muestreo e identificación molecular de las especies de hongos asociadas a la corchosis de la raíz del cafeto.
The plants were kept in a greenhouse for one year to obtain numerous corky-roots. The roots were washed with tap water to remove the excess of soil. Longitudinal cuts of corky-roots were made with scalpel, and 1 mm thickness rectangular sections (2 mm x 5 mm) of inner tissues of the corky-root parts were collected and fixed in glutaraldehyde at 2% for 5 days to preserve the structural integrity. Subsequently, the samples were submitted to a dehydration process with increasing concentrations of ethanol (10, 30, 50, 70 and 90% from 15 to 25 min in each concentration) until conserving the tissue root sections in absolute alcohol.
The samples were placed in a filter paper bag and dehydrated in a critical point camera. Then the samples were mounted on aluminum cylindrical stubs and coated with gold-palladium for its further observation under SEM. Root sections of healthy plants of same age (15 months) were collected and processed in the same way as controls.
Isolation and identification of fungi
For the fungi isolations, roots from the eight sampling sites, apparently with recently formed corky swelling, were selected to avoid saprophytic fungi that may be present in old corky-root formations. Roots were carefully washed with tap water to remove adhered soil, then disinfected by consecutively soaking in 70% alcohol (during 1 minute), 3% NaCIO (1 min), 96% alcohol (30 seconds); and then rinsed tree times with distillated sterile water. Longitudinal cuts of the corky-root tissues were made and fragments of the inner tissues were extracted and placed in Petri dishes prepared with potato dextrose agar (PDA) and chloramphenicol (1 mg mL-1).
Fungi mycelia that grew from the extracted inner part of corky-root tissue fragments were transferred to other Petri dishes with PDA, until pure cultures from each isolate were obtained. To identify the fungi at species level, DNA was extracted from 25 mg of mycelia of each strain using the extraction kit: Fungal/Bacterial DNA MiniPrep Zymo Research. A molecular marker of 500 bp, that encompasses the Internal Transcriber Spacer (ITS) 1, the 5.8 rDNA, and the ITS2 molecular markers, was used and amplified by PCR (34).
The PCR products were analyzed on a 1.2 % agarose gel; and the DNA was purified and sent to Macrogen INC for sequencing.
The obtained sequences were edited in the e-Biox program and compared by BLAST analysis to the database of the National Center for Biotechnology Information (NCBI).
Pathogenicity tests of fungi in coffee plants
The 27 Fusarium oxysporum strains previously isolated and identified were selected for pathogenicity test because they were the only species found at all sampling sites. The inoculum was prepared by culturing mycelium of each strain in flasks with oat-yeast extract (10 g L-1 and 1 g L-1) liquid medium. The flasks were incubated in an orbital shaker at 150 rpm and 25°C during 5 days. The conidia concentration was determined with a Neubauer chamber and was adjusted to 1.106 spores per mL.
Ex vitro plantlets of Coffea arabica with two or three pairs of leaves of a F1 intraspecific hybrid H18 (ET06 wild Ethiopian accession x introgressed Cv. Naryelis) were used for this experiment. Besides the fact that ex vitro plantlets acclimated in horticultural trays filled with sterilized peat-moss allowed working with pathogen-free vegetal material this germplasm micropropagated by somatic embryogenesis provided strongly homogeneous material (8). Plantlets were extracted from horticultural trays and roots were carefully washed in distilled sterile water. Two different groups of plantlets were prepared for F. oxysporum inoculation.
The first group of plantlets was predisposed to the fungus infection by cutting the roots 1 cm from their apex with a sterile scalpel (13, 35). In the second group, plantlets were kept with intact roots. Each strain of F. oxysporum was inoculated on 5 plantlets of each of the two groups by submerging rootlets in 75 mL of a conidia suspension for 20 min (17). In each case, a group of plantlets without F. oxysporum inoculation was used as control. Subsequently all coffee plantlets were sowed in 100 mL pots filled with a sterilized (twice autoclaving) peat moss-sand 2:1 mix and placed in a greenhouse at 25 ± 2°C with relative humidity of 80-90% a 12 hours photoperiod. The experiment was arranged under a completely randomized design. The plantlets were manually watered every 72 hours with sterile water.
45 days after the inoculation, the plants were extracted from the pots, 45 days after the inoculation. Roots were washed with sterile distilled water to remove the substrate. Symptoms like lesions, root necrosis and wilt were annotated through a scale from 1 to 5 in order to determine the severity rate of disease according to Parke and Grau (1993) and Reis and Boiteux (2007) where: 1 = Plant without symptoms; 2 = Plants without wilting symptoms, but with light brown spots on the root; 3 = Plants with vascular necrosis symptoms and wilting symptoms, but without yellowing of the leaves; 4 = Generalized necrosis in the root, wilting and severe chlorosis; 5 = Dead plant.
To detect the vascular colonization of the different F. oxysporum strains along the root and the stem, three plants of each strain were selected in each group of plants. The surface of both parts of the plantlets was subsequently disinfected with 70% alcohol (during 1 min), 3% NaCIO (1 min) and 96% alcohol (30 seconds); then rinsed three times with sterile distilled water.
The first 3 mm next to the collar plant cutting were removed from root and stem parts.
The remaining root and stem parts were cut over a 50 mm length from the base into 10 sections of equal length. Root and stem sections of each plantlet were placed horizontally clockwise arranged into 90 mm Petri dishes with PDA-Chloramphenicol.
The Petri dishes with root and stem sections were accommodated in the laboratory under a totally randomized design and were incubated at 25°C for 8 days and examined every day for outgrowths of the fungi from the vascular ring of each root or stem section. The mycelia that grew from the root or stem fragments was transferred to Petri dishes with PDA to obtain pure cultures and to molecularly identify them in accordance with the methodology described above. The depth or height reached by the fungus inside the root or stem was determined from the re-isolation data for the root or stem sections for each plant (35).
Data on symptoms and plant development were analized by one-way ANOVA for each group of plants (wounded and healthy roots). The data obtained from the frequency of re-isolation between the two groups of plants (wounded and healthy roots) and between plant organ (root and stem), were used to build a distance matrix, calculating statistical distances with the Bray-Curtis method. To assess the effect of the groups of plant and plant organ on the frequency of fungal re-isolation, the distance matrix was analyzed with a permutational analysis of variance (PERMANOVA). To compare the vascular colonization between each isolate, the data was evaluated as a function of the frequency of re-isolations in the stems and roots in the ten sections arranged from the base of the stem or root every 5mm up to 50 mm. Percentage values of re-isolations were submitted to one-way ANOVA.
RESULTS AND DISCUSSION
Coffee corky-root disease, tissue observations
Many females, eggs and second juvenile stage (J2) of M. paranaensis were observed in the roots affected with the coffee corkyroot disease (figure 1a, 1b and 1c, page 284).

Figure 1. Coffee corky-root disease symptoms associated to Meloidogyne paranaensis parasitism on Coffea arabica.
Figura 1. Síntomas de la corchosis de la raíz del cafeto asociada al parasitismo de Meloidogyne paranaensis en Coffea arabica.
Cell lesions caused by the movement of the J2 through the root tissues were also observed (figure 1b, page 284). Corky protrusions with presence of numerous M. paranaensis individuals (females, J2 and egg masses) were observed on the stem up to about 5 cm above ground (figure 1d, page 284). To our knowledge, this the first report of RKN presence and symptoms at this above ground distance in plant stem. The pericycle and cortical tissues of corky-roots and stem lower parts of infested coffee plants showed cell distortions and corrugations, as well as some cell wall thickenings (figure 1b and 1c, page 284; figure 2a and 2b, page 285).
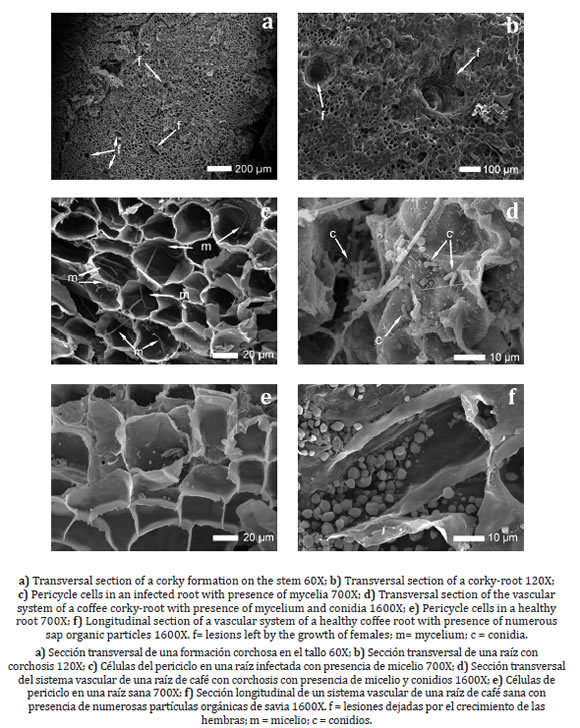
Figure 2. Sections of healthy and infested coffee roots and stems observed under scanning electron microscope.
Figura 2. Secciones de raíces y tallos de café sanos e infestados observados bajo microscopio electrónico de barrido.
No change in cortical cell volume was observed in corky-root tissues compared to healthy tissues, but hyperplasia like process was observed in cortical cell layers leading to a lateral expansion of the root or stem cortex. Presence of many conidia was observed in the cells of the infested tissues, as well as abundant mycelium crossing the cell walls, even in tissue areas where nematodes were not observed (figure 2c, d, page 285).
Many organic particles which nature was confirmed by energy-dispersive X-ray spectroscopy (72.1% C and 27.9% O) (figure 2e, f, page 285), were observed in the vascular system of healthy roots. This material was not observed in the vascular systems of diseased roots, revealing a dysfunction in the vascular nutrient transport. Numerous bacteria were observed in the corky root tissues (figure 3a, b) while no bacteria were observed in healthy tissues.
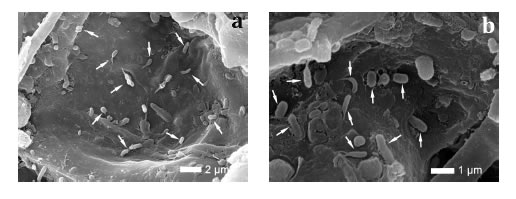
Figure 3. Bacteria inside cells of coffee corky-root tissues: a) 6000 and b) 13000X.
Figura 3. Bacterias dentro de las células de los tejidos de una raíz de café con corchosis: a) 6000 y b) 13000X.
To date no bacteria has been reported as associated to the coffee corky-root disease. However, after these observations it seems necessary to investigate if some of these bacteria detected in the inner corky-root tissues could be involved in the pathogenesis of the disease as being part of the corky-root pathobioma or if they just have an opportunistic role as saprophytes developing on decaying tissues. Studies on tomato indicate that the communities of endophyte bacteria are significantly affected by the infection of the nematode M. incognita bringing some new groups of bacteria, particularly those that contribute to the nematode infection process by degrading the plant cell walls or allowing a mutualistic relation with the provision of nutrients (37).
Diversity of fungi associated to coffee corky root disease
Forty-nine fungi strains were obtained from the coffee corky-root inner tissues. According to the molecular identification, 55% of the isolates correspond to Fusarium oxysporum; 12% to Penicillium citrinum; 10% to F. solani; 10% to Purpureocillium lilacinum; 4% to Alternaria longissima and the remaining 8% to the following species: Baeuveria bassiana, Gliocladiopsis curvata, Pochonia chlamydosporia and Stereum complicatum.
The isolation of all fungi for each sampling site is summarized in table 1 (page 281-282). Except for F. oxysporum and F. solani, all fungi species found in this work are registered for the first time on coffee corky-roots. Alternaria sp., B. bassiana, F. oxysporum and P. citrinum have been registered as endophyte of healthy coffee plant roots (30, 39, 40).
G. curvata is a fungus previously isolated from soil and plants debris, although its ecology or role as potential pathogen of plants is less known (23). S. complicatum is a saprophytic fungus commonly found in decaying wood tissues (3).
P. lilacinus and P. chlamydosporia are fungi commonly associated to nematodes. They may be found parasitizing M. paranaensis (19).
F. oxysporum was the only species found in all sampling sites of this study. In Costa Rica, the simultaneous role of F. oxysporum and the RKN, M. arabicida as causal agents of a similar coffee corky-root disease was demonstrated (4). In Puerto Rico, strains identified as F. oxysporum f. sp. coffeae, have been registered as pathogen, causing vascular wilting in coffee plants infested with the RKN, M. incognita, but without corky-root symptoms (27).
In Brazil, this same F. oxysporum f. sp. coffeae was reported for causing vascular wilting without the presence of nematodes (10). It has also been registered in abundance in the rhizosphere of coffee plants infected with the RKN M. exigua, without causing any symptoms of corkyroot or vascular disease. I has even been checked that some of this strains could have nematicide activity (15).
On the other hand, though F. solani has only been detected on four sampling sites, this fungus has also been previously detected in coffee corky-roots in the State of Veracruz (36). However, the only report of F. solani as a confirmed causal agent of a coffee disease is from Kenya, causing a coffee root rot (2). F. oxysporum and F. solani are considered separately as a complex of species including numerous plant pathogenic strains referred as special forms, related to some host plant(s); opportunistic strains that cause infections in humans and animals and saprophytic populations that are found commonly in soil, roots in senescence and vegetal debris (12, 38).
Pathogenicity and vascular colonization of Fusarium oxysporum on Coffea arabica plants.
Six weeks after inoculation, plants with the healthy and wounded roots did not show any wilting symptoms of wilting. Of the 1680 fragments examined in each group of plants, 354 re-isolations of F. oxysporum were achieved in plants with healthy roots and 288 in the plants with injured roots (0.06868, p = 0.001). No re-isolate was obtained from control plants. In both groups of plants, the fungi strains colonized the root, but not all the stems (0.11666 p = 0.001) and the re-isolation of the strains was discontinuous (figure 4 and 5, page 288).
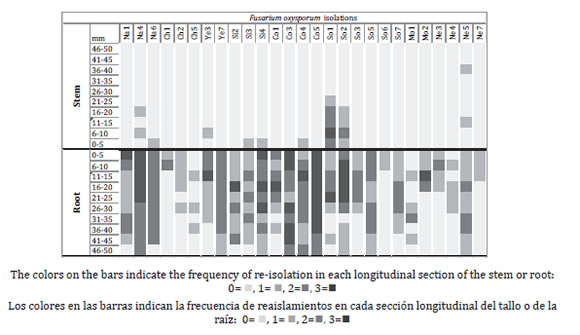
Figure 4. Vascular colonization of Fusarium oxysporum strains in root and stem, 45 days after inoculation in Coffea arabica plants with not wounded roots.
Figura 4. Colonización vascular en raíz y tallo de las cepas de Fusarium oxysporum 45 días después de la inoculación en plantas de Coffea arabica con raíces sin heridas.
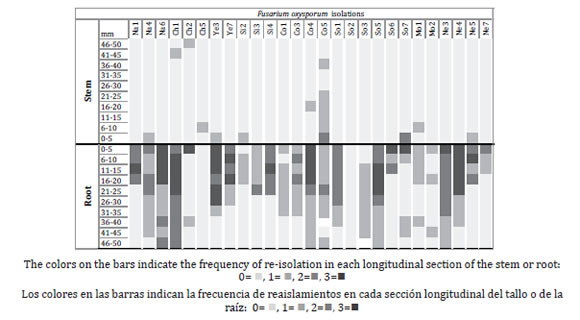
Figure 5. Vascular colonization of Fusarium oxysporum strains in root and stem, 45 days after inoculation in Coffea arabica plants with wounded roots.
Figura 5. Colonización vascular en raíz y tallo de las cepas de Fusarium oxysporum 45 días después de la inoculación en plantas de Coffea arabica con raíces con heridas.
All F. oxysporum strains were re-isolated in healthy roots, where 330 (93.2%) re-isolations corresponded to root and 24 (6.8%) to stems.
The strains Co5, Na4 and Co3 presented the highest (F = 7.8995, p = 0.00) frequency along the root with 93, 83 and 80% respectively. Ten strains of F. oxysporum were re-isolated from the stem of the plants with healthy roots.
The strain So1 presented the highest (F = 3.0639, P = 0.00) frequency of re-isolations in the stem (33%) to 30 mm hight, although the NE 5 strain was isolated at 40 mm from the stem base. In the plants with injured roots 25 of the 27 strains of F. oxysporum were re-isolated from the roots, 270 (93.75%) re-isolations corresponded to the root and 18 (6.25%) to stems.
The Ch1 and Na6 strains presented the highest (F=3.8618, P= 0.000) percentage of re-isolations in root, with 80 and 67%, respectively. Eleven strains of F. oxysporum were re-isolated from the stems of this group of plants with injured root. The Co5 strain presented the highest percentage of the re isolation (23%) to the height of 40 cm (F = 3.2517, P = 0.00). However, the maximum height was recorded for the strains Ch1 and Ch2 which were found at 45 and 50 mm from the stem base, respectively.
Different studies mention that the phytopathogenic fungi that causes withering, enter the roots by mechanic wounds, like the ones caused by nematode penetration (14) and in the case the RKN by the wounds caused by the expulsion of the eggs masses (18). Nevertheless, our results it is prove that this wounds are not necessary for F. oxysporum to colonize the vascular systems of coffee plants, since F. oxysporum isolated from corky root disease, colonized coffee plant roots with and without wounds.
All the studied strains colonized the root but only some got to the stem. The movement of the fungus to the stem is considered a pathogenicity indicator. Nevertheless, in this work, none of the isolations of F. oxysporum caused symptoms of vascular withering (9, 26, 32).
Some studies suggest that the phytopathogenic fungi can colonize their host and behave as endophytes long before presenting any symptoms of disease, which are expressed when the host plant goes into stress (33).
In coffee plants, it might possible that the strains of F. oxysporum present in the roots with the corky root disease enter the plant as endophytes and remain without causing any symptoms until the plant goes into any kind of stress (29). However this hypothesis must be studied.
CONCLUSIONS
According to the observations, tissues of coffee infested with corky-root diseases have nematodes, fungi and bacteria interacting at once. F. oxysporum is a major fungus associated with coffee corky-root disease.
F. oxysporum strains isolated from corky-root disease do not cause wilting symptoms in coffee plants in the absence of nematode M. paranaensis. Nevertheless, more studies in vivo and at molecular level should be conducted, detecting pathogenicity genes and determining if these strains are latent pathogens or saprophytes in coffee roots before being affected by nematodes.
1. Avelino, J.; Cristancho, M.; Georgiou, S.; Imbach, P.; Aguilar, L.; Bornemann, G.; Läderach, P.; Anzueto, F.; Hruska, A. J.; Morales, C. 2015. The coffee rust crises in Colombia and Central America (2008-2013): impacts, plausible causes and proposed solutions. Food Security. 7(2): 303-321.
2. Baker, C. J. 1972. Fusarium solani associated with a wilt of Coffea arabica in Kenya. East African Agricultural and Forestry Journal. 38(2): 137-140.
3. Berry, F. H.; Lombard, F. F. 1978. Basidiomycetes associated with decay of living oak trees. Broomall. USA. USDA Forest Service Research Paper. 8 p.
4. Bertrand, B.; Nuñez, C.; Sarah, J. L. 2000. Disease complex in coffee involving Meloidogyne arabicida and Fusarium oxysporum. Plant Pathology. 49(3): 383-388.
5. Bertrand, B.; Ramirez, G.; Topart, P.; Anthony, F. 2002. Resistance of cultivated coffee (Coffea arabica and C. canephora) trees to corky-root caused by Meloidogyne arabicida and Fusarium oxysporum, undercontrolled and field conditions. Crop Protection. 21(9): 713-719.
6. Bertrand, B.; Anthony, F. 2008. Genetics of resistance to root-knot nematodes (Meloidogyne spp.) and breeding. In: Souza, R. M. (Ed.). Plant-Parasitic Nematodes of Coffee. Brazil. Springer. 165-190.
7. Bertrand, B.; Alpizar, E.; Lara, L.; SantaCreo, R.; Hidalgo, M.; Quijano, J. M.; Montagnon, C.; Georget, F.; Etienne, H. 2011. Performance of Coffea arabica F1 hybrids in agroforestry and full-sun cropping systems in comparison with American pure line cultivars. Euphytica. 181(2): 147-158.
8. Bobadilla, L. R.; Cenci, A.; Georget, F.; Bertrand, B.; Camayo, G.; Dechamp, E.; Simpson, J.; Herrera, J. C.; Santoni, S.; Lashermes, P.; Etienne, H. 2013. Highgenetic and epigenetic stability in Coffea arabica plants derived fromembryogenic suspensions and secondary embryogenesis as eevealed by AFLP, MSAP and the phenotypic variation rate. PLoS One. 8(2): e56372, http://dx.doi.org/10.1371/journal.pone.0056372.
9. Bonacci, M.; Barros, G. 2019. Genetic diversity and pathogenicity on root seedlings from three soybean cultivars of Fusarium graminearum isolated from maize crop residues. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 51(1): 147-160.
10. Cardoso, R. M. L. 1986. A vascular wilt of coffee (Coffea arabica) in the state of Parana, Brasil, caused by Fusarium oxysporum f. sp. coffeae. Fitopatología Brasileira. 11(4): 753-760.
11. Carneiro, R. M. D. G.; Cofcewicz, E. T. 2008. Taxonomy of Coffee-Parasitic Root-Knot Nematodes, Meloidogyne spp. In:Souza, R. M. (Ed.). Plant-parasitic nematodes of coffee. Brazil. Springer. p. 87-122.
12. Chiotta, M. L.; Chulze, S.; Barros, G. 2015. Fuentes de inóculo de especies de Fusarium potenciales productoras de micotoxinas en el agroecosistema soja. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 47(2): 171-184.
13. Cumagun, C. J. R.; Aguirre, J. A.; Relevante, C. A.; Balatero, C. H. 2010. Pathogenicity and aggressiveness of Fusarium oxysporum Schl. in bottle gourd and bitter gourd. Plant Protect. Sci. 46: 51-58.
14. Francl, L. J.; Wheeler, T. A. 1993. Interaction of plant-parasitic nematodes with wilt-inducing fungi. In: Khan, M. W. (Ed.). Nematode Interactions. Springer. Netherlands. 79-103.
15. Freire, E. S.; Campos, V. P.; Pinho, R. S. C.; Oliveira, D. F.; Faira, M. R.; Pohlit, A. M.; Noberto, N. P.; Rezende, E. L.; Pfenning, L. H.; Silva, J. R. C. 2012.Volatile substances produced by Fusarium oxysporum from coffee rhizosphere and other microbes affect Meloidogyne incognita and Arthrobotrys conoides. Journal of Nematology. 44(4): 321-328.
16. García, P. P.; Cid del Prado, V. I.; Zavaleta-Mejía, E.; Téliz, O. D. 1997. La corchosis del cafeto (Coffea arabica L.) alternativas de su manejo. Nematropica. 27(2): 111.
17. Haegi, A.; Catalano, V.; Luongo, L.; Vitale, S.; Scotton, M.; Ficcadenti, N.; Belisario, A. 2013. A newly developed real-time PCR assay for detection and quantification of Fusarium oxysporum and its use in compatible and incompatible interactions with grafted melon genotypes. Phytopathology. 103(8): 802-810.
18. Haseeb, A.; Sharma, A.; Ahukla, P. K. 2005. Studies on the management of root-knot nematode, Meloidogyne incognita-wilt fungus, Fusarium oxysporum disease complex of green gram, Vigna radiata cv ML-1108. J Zhejiang Univ SCI. 6(8): 736-742.
19. Hernández-Leal, T.; López-Lima, D.; Carrion, G. 2016. Effect of the application of nematophagous fungus Purpureocillium lilacinum over nutrients availability on agricultural soil and yield of Avena sativa. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 48(2): 1-12.
20. Hernández-Martínez, G.; Manson, R.; Contreras-Hernández, A. 2009. Quantitative classification of coffee agroecosystems spanning a range of production intensities in central Veracruz, Mexico. Agriculture, Ecosystems and Environment. 134(1-2): 89-98.
21. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). 2005. Clones de Coffea canephora como patrones para injertos con tolerancia a la corchosis de la raíz del café. Folleto técnico no. 3. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Available in: http:// www.inifap.gob.mx/Documents/reportes/ reporte_ anual2004.pdf (Accessed: April 2016).
22. International Coffee Organization (ICO). 2016. Coffee trade statistics. Available in:http://www. ico.org (Accessed January 2017).
23. Lombard, L.; Crous, P. W. 2012. Phylogeny and taxonomy of the genus Gliocladiopsis. Persoonia. 28: 25-33.
24. López, R.; Salazar, L. 1989. Meloidogyne arabicida n. sp. (Nemata: Heteroderidae) nativo de Costa Rica. Un nuevo y severo patógeno del cafeto. Turrialba. 39(3): 313-323.
25. López-Lima, D.; Sánchez-Nava, P.; Carrión, G.; Espinosa de los Monteros, A.; Villain, L. 2015. Corky-root symptoms for coffee in central Veracruz are linked to the root-knot nematode Meloidogyne paranaensis, a new report for Mexico. European Journal of Plant Pathology. 141(3): 623-629.
26. Michielse, C. B.; Rep, M. 2009. Pathogen profile update: Fusarium oxysporum. Molecular Plant Pathology. 10(3): 311-324.
27. Negrón, J. A.; Acosta, N. 1989. The Fusarium oxysporum f. sp. coffeae-Meloidogyne incognita complex in Bourbon Coffee. Nematropica. 19(2): 161-168.
28. Parke, J. L.; Grau, C. R. 1993. Aphanomyces. In: Singleton, L. L.; Mihail, J. D.; Rush, C. M. (Eds.). Methods for research on soilborne phytopathogenic fungi. USA. APS Press. 27-30.
29. Peverelli, M. C.; Rogers, W. J. 2013. Heat stress effects on crop performance and tools for tolerance breeding. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 45(2): 349-368.
30. Posada, F.; Aime, M. C.; Peterson, S. W.; Rehner, S. A.; Vega, F. E. 2007. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycological Research. 111(6): 749-758.
31. Reis, A.; Boiteux, L. S. 2007. Outbreak of Fusarium oxysporum f. sp. lycopersici race 3 in commercial fresh-market tomato fields in Rio de Janeiro State, Brazil. Horticultura Brasileira. 25(3): 451-454.
32. Rodríguez-Molina, M. C.; Medina, I.; Torres-Vila, L. M.; Cuartero, J. 2003. Vascular colonization patterns in susceptible and resistant tomato cultivars inoculated with Fusarium oxysporum f.sp. lycopersici races 0 and 1. Plant Pathology. 52(2): 199-203.
33. Romero, A.; Carrion, G.; Rico-Gray, V. 2001.Fungal latent pathogens and endophytes from leaves of Parthenium hysterophorus (Asteraceae). Fungal Diversity. 7: 81-87.
34. Schoch, C. L.; Seifert, K. A.; Huhndorf, S.; Robert, V.; Spouge, J. L.; Levesque, C. A.; Chen, W. Fungal Barcoding Consortium. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS. 109(16): 6241-6246.
35. Sestili, S.; Polverari, A.; Luongo, L.; Ferrarini, A.; Scotton, M.; Hussain, J.; Delledonne, M.; Ficcadenti, N.; Belisario, A. 2011. Distinct colonization patterns and cDNA-AFLP transcriptome profiles in compatible and incompatible interactions between melon and different races of Fusarium oxysporum f. sp. melonis. BMC Genomics. 12:122.
36. Téliz-Ortíz, D.; Castillo-Ponce, G.; Nieto-Angel, D. 1993. La corchosis del cafeto en México. Revista Mexicana de Fitopatología. 11(1): 5-12.
37. Tian, B. Y.; Cao, Y.; Zhang, K. Q. 2015. Metagenomic insights into communities, functions of endophytes, and their associates with infection by root-knot nematode, Meloidogyne incognita, in tomato roots. Scientific reports. 5:17087.
38. van Diepeningen, A. D.; de Hoog, G. S. 2016. Challenges in Fusarium, a trans-kingdom pathogen. Mycopathologia. 181(3): 161-163.
39. Vega, F. E.; Posada, F.; Peterson, S. W.; Gianfagna, T. J.; Chaves, F. 2006. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia. 98(1): 31-42.
40. Vega, F. E.; Simpkins, A.; Aime, M. C.; Posada, F.; Peterson, S. W.; Rehner, S. A.; Infante, F.; Castillo, A.; Arnold, E. 2010. Fungal endophyte diversity in coffee plants from Colombia, Hawaii, Mexico and Puerto Rico. Fungal Ecology. 3(3): 122-138.
41. Villain, L.; Molina, A.; Sierra, S.; Decazy, B.; Sarah, J. L. 2000. Effect of grafting and nematicide treatments on damage by root-lesion nematodes (Pratylenchus spp.) to Coffea arabica L. in Guatemala. Nematropica. 30(1):87-100.
42. Villain, L.; Sarah, J. L.; Hernández, A.; Bertrand, B.; Anthony, F.; Lashermes, P.; Charmetant, P.; Anzueto, F.; Carneiro R. M. D. G. 2013. Diversity of root-knot nematodes parasitizing coffee in Central America. Nematropica. 43(2):194-206.
ACKNOWLEDGEMENTS
The authors of this study acknowledge to Greta Hanako Rosas Saito for the coffee root observations under the scanning electron microscope. We also thank Magda Gomez Columna for their technical support in the isolation and maintenance of fungal strains. This work was supported by the Consejo Nacional de Ciencia y Tecnología (grant number 231215).