REVISION
Opuntia ellisiana Griffiths as livestock feed in areas similar to USDA cold hardiness zones 6-7
Opuntia ellisiana Griffiths como alimento para el ganado en áreas similares a las zonas de resistencia al frío USDA 6-7
Josefina María Grünwaldt 1, Peter Felker 2, Juan Carlos Guevara 1, Eduardo Guillermo Grünwaldt 1
1 Argentine Institute for Arid Land Research (IADIZA-CCT-CONICET-MENDOZA) Av. Adrián Ruiz Leal s/n. Parque Gral. San Martín. 5500. Mendoza. Argentina. jgrunwaldt@mendoza-conicet.gob.ar
2 D' Arrigo Bros. Co. of California. 21777 Harris Road Salinas. California. 93908 USA.
Originales: Recepción: 19/05/2018 - Aceptación: 09/09/2018
ABSTRACT
The present review compiles the studies carried out so far on Opuntia ellisiana Griffiths. This species, of unknown origin, was first described at the beginning of the 20th century in southern Texas, USA, and introduced to Argentina in 1998. This species, like other Opuntia sps., can be cultivated in a wide range of environments and its lower transpiration per unit of carbon gained in relation to C3 and C4, has lead to in an important increase in water-use efficiency. While O. ellisiana has a lower growth and productivity than O. ficus-indica (L.) Mill. it stands out for its resistance to sub-zero temperatures. Fortunately, the intraspecies variation within O. ellisiana, shortens the time for its use after establishment. There is a wide variation in the nutrient content between the different forage species and clones of Opuntia. Due to the inherently low N availability in arid ecosystems, O. ellisiana, like the other species, has low protein content in natural unfertilized conditions. Some efforts, as the use of N-fertilizer, have been carried out to improve its protein level. About 15% protein levels have been obtained with other Opuntias. Other research has been directed to provide a favorable abiotic environment for a cactus to achieve higher biomass productivity and improved protein levels by interacting with nurse plants, such as Prosopis sps. The last alternative resulted in a significant increase in protein content and cladode quantity per plant of O. ellisiana.
Keywords: Opuntia ellisiana; Phylogeny; Ecophysiology; Cold hardiness; Productivity; Nutrient content
RESUMEN
La presente revisión compila los estudios realizados hasta el presente sobre Opuntia ellisiana Griffiths. Esta especie, de origen desconocido, fue descripta primeramente a comienzos del siglo 20 en el sur de Texas, EE.UU. y fue introducida a la Argentina en 1998. Al igual que otras Opuntia sps. puede ser cultivada en un amplio rango de ambientes y su transpiración más baja por unidad de carbón ganada en relación con plantas C3 y C4 conduce a un importante incremento en la eficiencia del uso del agua. Mientras que O. ellisiana tiene un crecimiento y una productividad más bajos que O. ficus-indica (L.) Mill. se destaca por su resistencia a temperaturas sub-cero. Afortunadamente hay variación intra especie dentro de O. ellisiana que se puede utilizar para acortar el tiempo de uso después de su establecimiento. Existe una amplia variación en el contenido de nutrientes en las diferentes especies forrajeras y entre los clones de Opuntia. Debido a la baja disponibilidad de N en los ecosistemas áridos, O. ellisiana, al igual que las otras especies, tiene bajo contenido de proteínas en condiciones naturales sin fertilización. Algunos esfuerzos, como el uso de N como fertilizante, se han llevado a cabo para mejorar su nivel de proteína, alcanzando niveles proteicos cercanos al 15% en otras Opuntias. Algunas investigaciones han sido dirigidas a proporcionar un ambiente abiótico favorable para el cactus para lograr una mayor productividad de biomasa y niveles mejorados de proteína mediante la interacción con plantas nodrizas, tales como Prosopis sps. La última alternativa permitió incrementar significativamente el contenido de proteína y la cantidad de cladodios por planta de O. ellisiana.
Palabras clave: Opuntia ellisiana; Filogenia; Ecofisiología; Resistencia al frío; Productividad; Contenido de nutrientes
INTRODUCTION
Cactaceae, have evolved to develop adaptive mechanisms that allow them to ensure their survival in highly hostile conditions and are now part of the natural environment and agricultural systems worldwide.
Plantations of drought-tolerant and water-efficient fodder shrubs, especially Opuntia species, have been established as buffer feed reserve, as a strategy to mitigate the effects of drought in animal production systems in various arid and semiarid areas of the world.
In this strategy the buffer reserve was aimed not only as "drought insurance" for inter-annual drought but also to bridge over a recurrent annual period of feed scarcity (40). Opuntia species have the ability to withstand prolonged drought, high temperatures, as well as wind and water erosion (26).
Cactus and other drought-tolerant and water-efficient fodder shrubs can survive under rainfall as low as 50 mm on a particular year, but with neither growth nor production. Mean annual rainfall of 100-150 mm corresponds to the minimum required to successfully establish rainfed plantations (41), provided soils are sandy and deep (42).
Within the Cactaseas family, the genus Opuntia is considered the one of greatest agronomic importance and there are many reasons behind its worldwide diffusion such as simple cultivation practices required to grow the crop; rapid establishment soon after introduction in a new area; ability to grow in very harsh conditions characterized by high or low temperature, lack of water and poor soil; possibility of massive propagation by in vitro culture of areoles when there is low availability of material for propagation; appreciated fruits; use of stems in the human diet and as forage for livestock; production of a wide range of industrial derivatives. These and other factors have contributed to such a wide distribution, from the regions of origin in Latin America to remote areas in different continents, cultures and traditions (38, 45).
Cacti have greater water-use efficiency due to Crassulacean Acid Metabolism (CAM) photosynthetic pathway (35, 52, 53) making them especially suited for forage productions in arid lands.
At the end of the 20th century, the area under cultivated Opuntia for forage was reported to be 900,000 ha, greatly surpassing the reported area for fruit (100,000 ha). The succulence and nutritive value of Opuntia make it a valuable emergency crop, permitting livestock farmers in Brazil, Mexico, South Africa and USA to survive prolonged and severe droughts (61).
In Argentina, the cultivated area of cactus is estimated at 10,000 ha for forage and fruit production (11).
The major limitation to cultivation of cactus in many areas of the world is severe cold winter temperature as occurs in the region of Mendoza, Argentina (26), northern Mexico (4), the Mediterranean Basin (42), the arid highland steppes of western Asia (43) and the south-western United States (59).
Opuntia ellisiana is a slower growing species compared to other Opuntia species such as O. ficus-indica. Nevertheless O. ellisiana is the only spineless Opuntia fodder species that is completely cold resistant in Texas (34) and in Mendoza, Argentina (30, 31).
This review reports the findings of the studies on Opuntia ellisiana Griffiths with respect to phylogeny, ecophysiology, cold hardiness, productivity and nutrient content, and its interaction with Prosopis sps. as a nurse plant.
PHYLOGENY
The first systematic collections, descriptions and field testing of Opuntias from Mexico and southwestern USA for fruit, forage and cold hardiness was conducted in the first few years of the 20th century principally by David Griffiths (21, 23, 24, 25) including a 1906' with guidelines on the use of cactus in animal feed (19).
Among Griffith's extensive work there is one about O. ellisiana (20). In 1915 this species was described in a similar manner as in 1910 (22). This description indicated "Plant spreading, ascending, laxly to compactly branched, 1-1.5 m high, and 1.25-2 m in spread of branch, depending upon moisture and fertility conditions; joints light, pale, glaucous, green, when young, but yellowish shortly after maturity, broadly obovate, about 20 x 24 cm, slightly elevated at areoles when young; areoles at first almost cottony white, turning gray, and finally black, small, 2-3 mm in diameter, after leaves have fallen and maturity has approached, made up of a central papillum in which the spicules are produced surrounded by a depressed groove separating it from the outer zone of gray or white wool; leaves long, prominent, circular in sections or slightly flattened, subulate, cuspidate, broadly arched backward, 12-15 mm in length; spicules light yellow, never prominent, scarcely visible, few and only 1 mm or less in length, scarcely distinguishable except by feeling from the central papillum of wool in which they are situated; spines entirely absent; flowers deep yellow, changing to orange, reddish when closed, some of the outer perianth segments dull, greenish red in bud, about 6 cm in diameter when open, filaments and style white, stigma very light greenish yellow, 7-parted; fruit pyriform to hemispherical, deep reddish purple throughout, young ovary thickly beset above with small white subcircular areoles 3 mm apart, and 1.5 mm in diameter, the wool being prominently raised to 1 mm or more in a compact columnar tuft, from center of which are produced 1-2 delicate yellowish fugaceous spines, 2-3 mm long and 1-3 or 4 minute spicules 1 mm long or less, the lower part of ovary having only 1-3 spicules, and the areoles being much farther apart".
"The origin is not known, but it has evidently been in cultivation for a long time. It is now quite widely distributed in collections due to the efforts of the Department of Agriculture (Washington, D. C.) and Professor J. C. Ellis, who first found it cultivated by Mexicans in the outskirts of Corpus Christi. There are indications that it has been derived by selection from native forms of southern Texas; but the evidence is not conclusive. It is perfectly hardy at Austin, and as Op. cacanapa, and possibly as hardy as Op. subarmata (22). In growth it is not as fast as the other two; but it is much more smooth, approaching if not quite equaling in this respect, the smoother forms of the Indian-fig group. Another feature is lack of spicules on the fruits.
On this accounts, the species is quite promising for breeding purposes. While these three forms appear to be the most promising, and are the ones upon which the greatest effort is being expended at present, it is not at all impossible that other selections may be made of as great, if not even greater merit. One nearly spineless form recorded under my collection N° 9087, from Webb County, Texas, is a rapid, very succulent, wavy jointed, compact form, as good as any of the above, were it not for its few spines. It is probably very close to, if not the same as, forms of Opuntia subarmata. Another selection made last year is a remarkably smooth form of Op. bentonii. It is thus far devoid of spines, but has quite prominent spicules. This grows rapidly, but its joints are as thin as those of Op. cacanapa. The difference in cold resistance of these forms is not great. They will withstand from -6.7 to -11.1°C lower temperatures than the conventional spineless ones of today; and will probably all be hardy throughout the entire pear region of Texas" (22).
In 1919, O. ellisiana is mentioned as an unarmed species and known only from cultivated plants. It was stated by Griffiths that it is quite different from the Ficus-indicae series, which in much resembles, and is quite hardy in southern Texas. It may be a spineless race of the common O. lindheimeri of this region (6).
The taxonomic instability of the Opuntioideae is not restricted to the generic classification. Opuntia sps. in particular, the largest genus in the subfamily, is well known for being extremely difficult taxonomically at the species and lower levels as a consequence of a high incidence of inter specific hybridization and polyploidy, which have resulted in complex patterns of morphological variation.
O. ellisiana is mentioned as a species distributed and exclusively cultivated in the USA (35).
Wild Opuntia (62, 71) can be diploid, triploid, tetraploid and octaploid (n = 11). Evidently due to insect pollination of Opuntia’s perfect, self-fertile flowers, today’s commercial O. ficus-indica land races for fruit use are octaploid (60). While a diploid spineless O. ficus-indica in Alpine, Texas (71), had been reported the species designation for this accession was later determined to be O. ellisiana. A later work confirms the diploidy of O. ellisiana: "Of the 164 species in the Opuntieae for which chromosome counts have been carried out, including our new counts, 26.2% are diploid, 13.4% are both diploid and polyploid, and 60.4% are polyploid reiterating that the frequency of genome duplication in the group is far more common than diploidy" (46).
Among other characteristics, phenotypically, O. ellissiana can be differentiated from O. ficus-indica by its lower height at maturity (1.0-1.5 m vs. up to 6 m); by the length of its cladodes (20 cm vs 20-50 cm) and by the shape (pyriform to hemispherical vs tuberculate, ovoid to oblong) and color of its fruits (dark red to almost purple vs yellow, orange, pink-green or reddish), respectively.
Texas A&M University, Kingsville, (TAMUK) has been involved since 1982 in collection and introduction of Opuntia to the USA, as well as agronomic research and extension (49). The programme focuses on the development of freezetolerant cultivars, as freeze damage is a common problem in the region (70). In 1996, the first round of crosses marked the beginning of a long-term breeding programme. In 1998, the genetic material, including O. ellisiana clone 1364, was transferred to National University of Santiago del Estero, Argentina by Peter Felker (pers. comm. 2018). At present, O. ellisiana existing in the Mendoza province descends from the clone 1364, taking part of research works for its use as fodder.
ECOPHYSIOLOGY
Crassulacean acid metabolism (CAM) photo-synthesis is known in 33 families with an estimated 15 to 20,000 species, including O. ellisiana. These CAM plants express the most plastic and tenacious photosynthesis known. They can switch photosynthesis pathways and live and conduct photosynthesis for years even in the virtual absence of external H2O and CO2, i.e., CAM tenaciously protects its photosynthesis from both H2O and CO2 stresses (3).
CAM metabolism is one of the three metabolic pathways found in the photosynthetic tissue of vascular plants for assimilation for atmospheric CO2. Elucidation of the complete pathway of carbon assimilation in CAM plants took nearly 150 years and encompassed many fundamental discoveries in plant biochemistry (73).
To understand CAM photosynthesis, several landmark discoveries were made at the following times, i.e., daily reciprocal acid and carbohydrate cycles were found during 1870 to 1887; their precise identification, as malic acid and starch, and accurate quantification occurred from 1940 to 1954; and photosynthesis in two different types of cells was discovered from 1965 to approximately 1974. Therefore, by approximately 1980, CAM photosynthesis was finally rigorously outlined (3).
The physiological basis of the ecological success and agricultural usefulness of opuntias as a forage, in large measure reflects their daily pattern of stomatal opening. Most plants have daytime stomatal opening so that CO2 uptake occurs concomitantly with photosynthesis, which uses the energy of light to incorporate CO2 from the atmosphere into carbohydrates. Plants like opuntias, however, have nocturnal stomatal opening, so net CO2 uptake and water loss occur during the cooler part of the 24-hour cycle (55) and like other CAM plants, accumulate and store malate in the vacuoles of the chlorenchyma cells. This gas exchange pattern is referred to as CAM because it was studied extensively in the Crassulaceae, although apparently first recognized in the Cactaceae (51, 68). In O. ficus-indica, net nocturnal CO2 uptake had a relatively low optimal temperature, ranging from 11°C for plants grown at day/night air temperature of 10°C/0°C to 23°C at 45°C/35°C. Nocturnal CO2 uptake and acid accumulation summed over the whole night were maximal for growth temperatures near 25°C/15°C, with CO2 uptake decreasing more rapidly than acid accumulation as the growth temperature was raised (57).
CAM plants are native to arid and semi-arid regions, as well as to periodically dry microhabitats such as those occupied by epiphytes. Most of the 20,000 species of CAM plants are epiphytes growing on trees in tropical forests (52, 55, 72).
Since night-time temperatures are lower than diurnal ones, and relative humidity is generally higher, the transpiration of CAM plants is three to five times lower than that of C3 and C4 plants. The result is a tremendous increase in water-use efficiency and in the plant's ability to thrive in semi-arid environments characterized by a restricted water supply (200-300 mm of annual rainfall) or where long periods of drought and relatively high temperatures occur.
The mechanisms of adaptation to aridity are not necessarily valid in relation to high temperatures. Although they occur at night, CO2 uptake and acid accumulation are strongly influenced by environmental variables such as air temperature, light, plant water status, nutrients and soil salinity (51). There are plantations in Aziza (Lybia) where the maximum temperature exceed 50°C (44). Cladodes of different opuntias species, included O. ficus-indica, cannot survive between 64 and 70°C (56). In Mexico the regions of greatest diversity for commercial fruit varieties are in the altiplano of central Mexico averaging 1,800 m elevation where the maximum daily temperatures are much lower than the lower elevations of north eastern Mexico.
The daily pattern and the magnitude of total net CO2 uptake by O. ficus-indica mainly reflect nocturnal temperatures. Most cacti examined have a low temperature optimum (near 15°C) for net CO2 uptake. Moreover, substantial net CO2 uptake occurs at 0°C for O. ficus-indica, and O. humifusa can even have substantial net CO2 uptake at air temperatures of -5°C. Thus, low nighttime temperatures are not disadvantageous for net CO2 uptake by these cacti, whereas high nighttime temperatures, such as those above 30°C, can lead to appreciable stomatal closure and hence limited net CO2 uptake, leading to poor plant growth and production and eventually to low crop value (58).
Cladode succulence acts as a buffer to maintain turgor in the photosynthetic tissue (chlorenchyma), making it possible for the cladode to continue photosynthesizing during dry periods (37). Daily fluctuations in cladode thickness may also represent an early indicator of dehydration stress. Young cladodes show more pronounced diel thickness fluctuations compared with older cladodes, and therefore serve as a suitable model for assessing plant responses to environmental factors. Under well-watered conditions, diel fluctuations of cladode thickness are directly related to temperature variations, but not under severe drought stress (65).
Water-use efficiency (WUE) has been defined as the amount of water used per unit of plant material produced (5). This definition may be too broad in that it embraces water-use efficiencies obtained in diverse time and process scales. The plant material can be expressed as carbon dioxide assimilation, total biomass, or yield; the water use can be expressed as transpiration, evapo-transpiration or total water input to the ecosystem; and the timescale can be instantaneous, daily or seasonal (66). These authors stressed that water-use efficiency obtained at different time and process scales should not be used interchangeably.
It has been widely accepted that, in general, plants possessing CAM have higher water-use efficiency than C3 and C4 plants. The transpiration ratio for CAM plants ranged from 50 to 125 kg H2O/kg CO2, while for C3 plants: from 450 to 950 kg H2O/kg CO2 and for C4 plants: from 250 to 350 kg H2O/kg CO2 (2). As nearly all of the 130 accessions of germplasm collection (34) have greater height growth than Opuntia ellisiana under the same rainfall conditions, it would appear that they might have even greater water-use efficiencies. These accessions are mainly represented by O. ficus-indica; O. lindheimerii and O. megacantha, from Mexico, Africa, USA, Chile and Brazil (P. Felker, pers. comm. 2018). The average WUE of O. ellisiana was 162 kg H2O DM-1. This is among the highest WUE of any plant species, including C3 and C4, measured under long-term field conditions. Considering that WUE measured for O. ficus-indica was 250-300 kg H2O DM-1 (41), WUE of O. ficus-indica is about 55 to 85% lower than O. ellisiana. Thus, it can be assumed that the lower productivity of O. ellisiana could be explained by its higher transpiration rates.
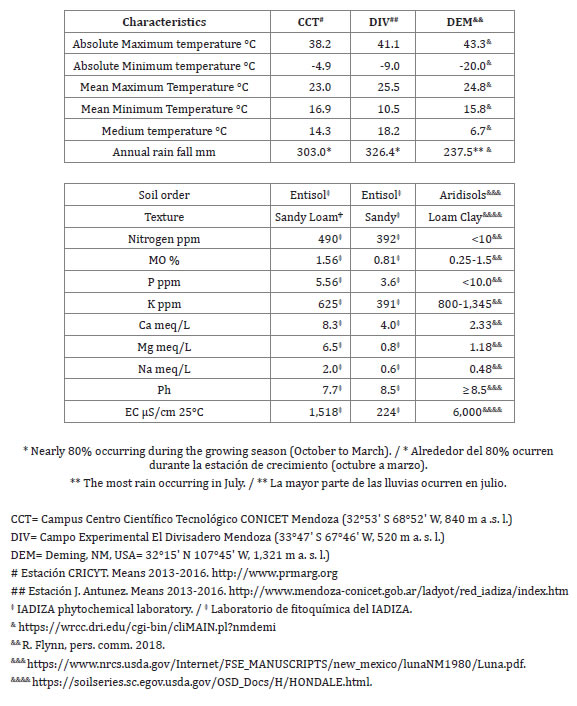
Table 1 (page 380), presents the climatic and soil characteristics of three sites in which experiments with O. ellisiana have been carried out: CCT CONICET (CCT), El Divisadero Cattle and Range Experimental Station (DIV) both in Mendoza, Argentine, and Deming (DEM) New Mexico, USA.
Table 1. Climatic and soil characteristics in the CCT CONICET (Mendoza, Argentine), El Divisadero Cattle and Range Experimental Station (Mendoza Province, Argentine) and Deming (New Mexico, USA).
Tabla 1. Características climáticas y de suelo en CCT CONICET (Mendoza, Argentina), Estación Experimental de Ganado y Pasturas Naturales El Divisadero (provincia de Mendoza, Argentina) y Deming (New Mexico, USA).

In the Mendoza plain, the dry season coincides with a cold winter, while the hot season, during which fruit and vegetative growth take place, correspond to the rainy season; whereas in Deming, NM; USA the dry season coincides with the hottest days, when the fruit develops and vegetative growth occurs.
The climatic conditions of the CCT, DIV and DEM are typical of arid zones with torrid summers, cold winters and great daily thermal amplitude, although in Deming it may be noted that the minimum absolute temperature is significantly lower, whereas no major differences in annual rainfall are among the three mentioned sites.
Soil composition presents its differences mainly in relation to nitrogen content and electrical conductivity; indicating that O. ellisiana has the capacity to develop under different soil characteristics.
COLD HARDINESS
During 1989, members of the Texas Prickly Pear Council discovered in Kingsville Texas, a thornless Opuntia that had survived a -12°C freeze without damage, when all O. ficus-indica, O. robusta types and others spineless types froze to ground level. This freeze hardy Opuntia appears to be O. ellisiana Griffiths (18). This species could be a useful forage variety in locations that are too cold for O. robusta Wendl. or O. ficus-indica (34). Other authors found that O. ellisiana was also completely tolerant to 20 hours below -7°C, with a minimum of -16°C (12). Although spineless varieties generally have less tolerance to freezing weather than spiny varieties (13), O. ellisiana suffered no damage when temperatures of -20°C were reached in a site located about 500 km north of Kingsville (70).
The experiments with Opuntia sps. as a fodder crop for drought periods began in the Mendoza plain, mid-west Argentina, at the end of 1995. The major limitation for cultivating this sort of cactus in this area, is the cold winter temperatures. Tolerance to sub-zero temperatures depends on the turgidity of cladode's chlorenchyma tissue, dehydration augmenting frost tolerance (42), while the relationship between water content of the cladodes and frost damage in general, showed no definite pattern (28).
When night temperatures in El Divisadero Cattle and Range Experiment Station field trial dropped to -12.3°C in the fall 1996, almost all the 7-month-old plants of some species of Opuntia were affected by these low temperatures. Thereafter, at the same study site, an Opuntia sps. collection was established containing the specimens that had survived the 1996 freeze and others new accessions with potential for increased cold hardiness. When night temperatures dropped to -16 and -17°C on 2 consecutive days in August 1999, great variability in frost damage was observed (28). A similar situation was found in Algeria where O. ficus-indica clones were severely damaged, after many genotypes were submitted to night temperatures of -8 to -10°C for a week, with midday temperatures rising to around 5°C (39). Cold hardiness of Opuntia sps. clones used for fruit, forage or vegetable production has been reported (18, 39, 59, 64, 70). Opuntia ellisiana in Texas endured a -9°C without apparent damage (64).
In El Divisadero Cattle and Range Experimental Station, one-year-growthperiod plants of O. ellisiana obtained by micropropagation (38) under 23.5 hours of temperatures ≤ -10°C in 2000 suffered no frost damage. The next year, the 2-yeargrowth- period plants suffered frost damage of about 0.9% under 19 hours at ≤ -10°C, 11 hours at ≤ -12°C and 5 hours at ≤ -13°C, indicating that it would not be necessary to protect the plants during winter for 1 or 2 years after planting. At the same time, O. ficus-indica suffered frost damage of 58.6%, statistically significant with respect to O. ellisiana, whose pads showed only slight necrosis around the margins. Frost damage for the 2-year-growth-period of O. ellisiana was significantly lower than for the 3-yeargrowth- period plants of O. ficus-indica and O. spinulifera from adjacent plantations.
The frost damage differences among O. ellisiana and the other two species would have been greater at a comparable plant age (30). In fact, frost damage proved to be inversely related to the age of the plant (28, 70). In the summer of 2016, Cushman of the University of Reno Nevada established a planting of ten spineless cold hardy progenies of O. ficus-indica x O. lindheimerii and O. ellisiania on the grounds of the University Campus. After the winter of 2016-2017 none of the cold hardy progenies of O. ficus-indica x O. lindheimerii survived while O. ellisiana survived with no damage. Temperature data for December, January and February found no temperatures as low as -12°C but recorded many days with temperatures of -5°C all day round. It seems that many days of below freezing weather, not necessarily reaching to an absolute minimum of -12°C, are lethal for the cold hardy forage clones (J. C. Cushman, pers. comm. 2018).
During the May-September 2009 period, the total hours with temperatures below 0°C and the absolute minimum temperatures in Ñacuñán (34°03' S, 67°58' W, similar to the study site: El Divisadero Cattle and Range Experimental Station), were 6 h and -1.8°C; 77 h and -7.1°C; 146 h and -6.1°C; 37 h and -4.7°C, and 54 h and -4.7°C in May, June, July, August and September, respectively. O. ellisiana suffered zero frost damage during this period (31).
Plants of O. ellisiana and O. ficus-indica, both obtained by micropropagation, were also established in Malargüe, Mendoza (35°28'8'' S, 69°35'07' W) in 1999. At this site, the mean daily minimum temperature of the coldest month is -4°C (29). Plants of O. ficus-indica froze to ground level while plants of O. ellisiana suffered no damage when temperatures dropped to -15°C in the winter of 2000 (30).
In an experiment carried out at the CCT Campus during 2014-2015, no frost damage was observed in the O. ellisiana cladodes, but it should be considered that winters of that period were not very rigorous, with a minimum temperature of -0.7°C (27).
Cladodes established in two places 5 km apart, with the same soil characteristics, a plain and a sand dune (10-15% slope) with north exposure, suffered frost damage that tended to be higher in the plain than in the dune. This difference can be explained by the cold protection provided by the dune (28). The latter could be taken as a recommendation for the implantation of O. ellisiana and other species in cold areas.
To arrive at a quantitative measure for freeze hardiness in perennials such as cacti, is difficult. Absolute minimum temperatures do not seem to be reliable indicators of freeze hardiness. Probably, a survey based on the average annual extreme minimum temperature during a 30-year period in the past, is more important than the lowest temperature ever occurred. Many plants that can survive a short period of exposure to cold, may not tolerate longer periods of cold weather. Therefore, using the USDA cold hardiness zones as a ranking criteria is advisable, given that most of the international botanical publications refer to them. Many other environmental factors, in addition to hardiness zones, contribute to the success or failure of plants such as light, soil moisture, temperature, humidity and duration of exposure to cold. The cold hardiness zones classification of some sites in which experiments with O. ellisiana have been carried out are 7 in Nevada, USA (69) and between 6 and 8 for Mendoza Province, Argentina (16).
It has been reported that in Deming, New Mexico, USA, USDA cold hardiness zone 7 (68), O. ellisiana never freezes. Clone 1364 of O. ellisiana was also not damaged by the worst freeze over 15 years in San Angelo, Texas, USA. Therefore it can be recommended for cold hardiness zone 7.
The adaptability of O. ellisiana to USDA cold hardiness zone 7 has important international ramifications. In North America, USDA cold hardiness zone 7 extends from the high elevation Chihuahua Desert of Mexico in the south, westward to southern Nevada and eastward through southern New Mexico, northern Texas, the states along the Gulf of Mexico and as far north as North Carolina. Tunisian researchers have obtained clones for testing in the foothills of the Atlas Mountains. Climates with USDA zone 7 are also located in the arid regions of southern Asia such as the foothills of the Himalayas in India and Pakistan (13). In Argentina, USDA cold hardiness zone 7 includes locations such as La Quiaca, Santa Rosa, Neuquén, Río Colorado, Villa Reynolds and Ushuaia (16).
PRODUCTIVITY AND NUTRITIVE VALUE
The production of O. ellisiana to O. ficus-indica ratio ranged from about 0.30 - 0.35 (1, 34) to 0.5 (H. Le Houérou, pers. comm. 1995). While O. ellisiana is not economically promising as it does not produce edible fruit and presents insufficient immature growth on a year-round basis to be a vegetable (nopalito) variety (34), it is an encouraging species to be used as forage in cold areas.
In Mendoza, Argentina, after a 2-year growth period, O. ellisiana plants obtained by micropropagation, established at 1 m x 5 m spacing, had reached 20.9, 22.2, and 24.8 cm in height under no irrigation, with 30 mm every 30 days and 15 mm every 15 days respectively, totaling 150 mm during the whole growing season. Plant height for the 30 mm every 30 days treatment was significantly higher than that for the no-irrigation treatment. However, irrigation did not significantly affect above-ground biomass production (g DM plant-1) and stem-area index (SAI) (cm2 plant-1). Nevertheless, dry-matter production and SAI tended to be higher in the irrigated plots than in the non-irrigated ones. Total biomass production (kg DM ha-1) was 146, 157, and 208 for no irrigation, 15-15 and 30-30 treatments, respectively (30). These dry-matter productions were obtained with a total water input during the growing season of 383 mm in 1999-2000 (150 mm irrigation plus 233 mm rainfall) and 369 mm in 2000-2001 (150 mm irrigation plus 219 mm rainfall).
The 2-year-growth-period average production (170 kg DM ha-1) represented only 2.8% of the production for 2-yeargrowth- period plants of O. ellisiana established at 1.5 x 1 m spacing in Texas (34). Several hypotheses could be advanced to explain this great difference in biomass between the two field trials. First, the Texas plantation was fertilized annually to avoid fertility limitation on water-use efficiency, while also receiving 1,691 mm rainfall in the 2-year growth period. In contrast, the plantation of the study carried out in El Divisadero Cattle and Range Experiment Station, was not fertilized and it received 700 mm rainfall plus 300 mm irrigation from the establishment date to the date when the biomass production was estimated. Second, the planting material probably had lower size (in terms of cladode dry weight) than the single cladodes used (34) and hence, the number and size of the shoots produced during the first year of growth in the field were lower in the plantation (48). Third, the low biomass from plants after the 2-year growth period seems to be due to a lower SAI that reached only 0.028, 0.029, and 0.038 for no irrigation, 15-15 and 30-30 treatments, respectively, while the Texas plantation had and SAI of 0.39 for the 2-year growth period (34). These authors found that biomass productivity was very low until a SAI of 2 was reached. At a SAI of 4 to 5 productivity of O. ficus-indica considered maximal (54).
Opuntia has low height growth compared to grasses, e.g., O. ellisiana only grows about 40 cm per year and O. ficus-indica only about 100 cm per year (34). However, due to annual extension of as many as 100 cladodes of 1.5 kg fresh weight in average (ca. 150 g dry weight) over the surface of several year old plant, productivity can be higher. For example, when O. ellisiana achieved a leaf area index of 2, it had a dry matter productivity of 17 Mg ha-1 yr-1 with only 662 mm rainfall (34). However at 4 yr with 38 dry t per ha, the height of this stand was only about 1 m. With a typical composition of 90% water (fresh weight basis), 6% protein (dry weight basis), 4% calcium (dry weight basis), 75% in vitro dry matter digestibility, and 72% digestible protein, cactus offers a highly digestible source of energy, a rich source of calcium for lactating animals, and high water content to offset the animal drinking requirements during drought periods. Indeed, the yearly fresh weight of 194,187 kg ha-1 in the fourth year would be sufficient for 4,315 cows day-1 at a feeding rate of 45 kg day-1 (12).
For O. ellisiana with slow height growth, grazing could not begin before the third year. Subsequent grazing is possible at 1 to 2 yr intervals depending on rainfall and weed control (13).
There is considerable variation in nutritional quality of Opuntia forage for various species or clones, growing conditions, and cladode ages (15, 17, 28, 50).
In arid conditions, the low quality of the forage and water shortage could be attenuated by the introduction of Opuntia species. In this regard, there is evidence of the beneficial effect of the inclusion of cactus in the ruminant´s diets. Cladodes contain high soluble carbohydrate, calcium and carotene contents, while they are low in protein and fiber (27, 32).
Some parameters related to nutritional quality of the 1-year-growth-period cladodes of O. ellisiana obtained by micropropagation expressed in percentage of dry matter (DM), were: crude protein (CP), 5.8; in vitro dry matter digestibility (IVDMD), 78.3; ash, 17.3 and organic matter (OM), 82.7 (30). Crude protein content of O. ellisiana cladodes was similar to that of 1-year-growth-period cladodes of O. paraguayensis (currently O. elata), O. ficus-indica and O. robusta clones growing in the same conditions in the study site, whose average was 5.9% (28). These protein values are somewhat higher than 4.1%, as the mean found for O. robusta, O. lindheimerii and O. ficus-indica in Mexico (15).
The IVDMD was high and similar to the overall value reported for opuntias. Given its low protein content, to supplement animal rations based on unfertilized cactus with protein, mineral, and vitamin supplements, such as soybean or cotton seed meal (12).
Opuntia interaction with nurse plants, such as Prosopis sps., have been directed to improve the cacti protein level The adaptation of Prosopis to herbivory is the main reason for its dominance in silvopastoral systems in arid and semiarid areas of America. Several species grow well usually under Prosopis canopy, responding to a higher soil nutrient content. O. ellisiana was implanted under and outside the canopy of isolated Prosopis sps. in Mendoza Province (32°53'45" S, 68°52'28" W, 840 m a. s. l.). After one year, the totality of cladodes was harvested. Frost damage was not observed under, nor outside the canopy (27).
Under the Prosopis canopy, the nutritional values of O. ellisiana were increased; nitrogen in particular, doubled its value. Productivity of cladodes per plant and concentrations of moisture, OM, acid detergent fiber, neutral detergent fiber, K and P in the cladodes were also significantly higher under Prosopis, while Ca and Na were higher outside the canopy. Magnesium values were not affected by the position (table 2, page 385).
Table 2. Means and standard deviations of bromatological values and yield of cladodes per plant of O. ellisiana planted outside and under Prosopis canopy in Mendoza Province, Argentine.
Tabla 2. Medias y desviaciones estándar de valores bromatológicos y producción de cladodios por planta de O. ellisiana bajo y fuera de la canopia de Prosopis en la provincia de Mendoza, Argentina.
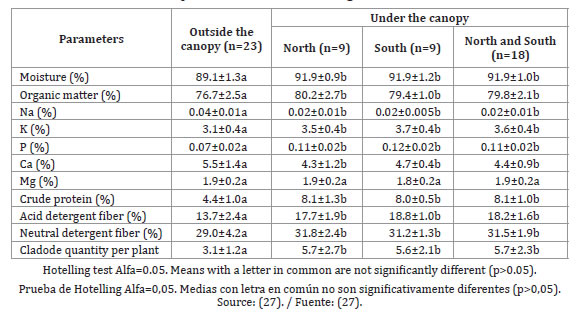
Under the crown of the tree, effective precipitation is greater than outside of it, due to the significant runoff of the branches and trunk (36), which favors the leaching of Na in the soil; while an increase in N and P reduces the uptake of Ca and Na (9).
The improvement in the forage value of this cactus under the Prosopis crown evidences the better condition of the site as a result of the higher nutrient content of the soil and the contribution of OM as mulch, resulting in the formation of fertility islands (63). In arid and semiarid ecosystems, dominant woody plants are likely to cause changes in microclimate and soil properties by mitigating harsh environmental conditions (e.g., high temperature and radiation) and by modifying soil characteristics, resource availability (e.g., water and nutrients) and spatial distribution of nutrients (7, 33).
The nurse effect of Prosopis improves nitrogen content of cladodes in the same way as cattle manure applied to soil (10). High doses of applied chemical fertilizers almost doubled the CP mean content of the 1-year-old cladodes when compared with the treatment in which no fertilizer was added: 7.8 and 4.3% DM, respectively (31).
Even though all the aforementioned, Cactus growing under Prosopis would die due to shade. In Texas, Opuntia sps. never grows under the canopy of mature forest but forms extensive growth just outside of it, if that area is cleared giving access to high levels of sunlight (P. Felker, pers. comm. 2018). However, other authors cite that Prosopis coverage facilitates the establishment of different species that do not settle in exposed areas (47). It has been suggested that the relationships of cacti and nurse species are primarily the result of intricate biotic relationships rather than differences in simple physical conditions (67). Strong interactions between soil and shaded effect were found when soil beneath Prosopis articulata shade, increased the biomass of Pachycereus pringlei (8).
POSSIBILITIES FOR GENETIC IMPROVEMENT
Crosses among many accessions of O. ficus-indica and between O. ficus-indica and O. lindheimerii have been examined (70). However, unsuccessful attempts in crossing O. ellisiana with O. lindheimerii in the Kingsville collection were carried out. It was later found that O. lindheimerii was hexaploid. Since O. ellisiana is a diploid, this should be expected. Much in the same way as (14), crossed O. lindheimerii with O. ficus-indica and obtained spineless progeny with much more cold hardiness than any O. ficus-indica, it would be very interesting to cross O. ellisiana with a faster growing diploid Opuntia. A spiny diploid O. lindheimerii that has been found (R. Puente, pers. comm. 2018) would be very interesting to cross with O. ellisiania. To completely eliminate spines on the progeny, it might be necessary to make an additional back cross to the O. ellisiana parent. As the estimated time expended from making a cross till evaluation of a 80 cm tall seedling, and subsequent additional backcrossing is on the order of 3-4 years, this is highly feasible.
CONCLUSIONS
Although Opuntia ellisiana with respect to other opuntias is not economically promising as a producer of forage, fruit and vegetables as other opuntias, it could be a useful forage variety in locations that are too cold, as evidenced by the results found in Argentina and the USA. Besides, this species has demonstrated its hardiness for its cultivation under different conditions. The results obtained from the association of O. ellisiana with Prosopis sps. are also encouraging in relation to the increase of cladode productivity per plant and important nutrient content improvement, like protein.
Plants of O. ellisiana obtained by micropropagation appear to be tolerant to freezing temperatures attained in areas with extremely cold winters, and in contrast with other Opuntia sps., to protect the plants in winter for 1 or 2 years after planting, seems to be unnecessary.
The little available information on nutrient content of O. ellisiana should inspire further research, considering that it has a promising future as a fodder in cold areas.
1. Barrientos-Pérez, F.; Borrego Escalante, F.; Felker, P. 1992. Collaborative Mexico/United States initiative to breed freeze tolerant fruit and forage Opuntia varieties, in: Felker, P. (Ed.). Third Annual Prickly Pear Council. Texas A&M University. Kingsville. Texas. p. 49-55.
2. Black, C. C. Jr. 1973. Photosynthetic carbon fixation in relation to net CO2 uptake. Annual Review of Plant Physiology. 24: 253-286.
3. Black, C. C.; Osmond, C. B. 2003. Crassulacean acid metabolism photosynthesis: ‘working the night shift’. Photosynthesis Research. 76: 329-341.
4. Borrego-Escalante, F.; Murillo-Soto, M. M.; Parga-Torres, V. M. 1990. Potencial de producción en el norte de México de variedades de nopal (Opuntia spp.) tolerantes al frío, in: Felker, P. (Ed.). Proceedings of the First Annual Texas Prickly Pear Council. Caesar Kleberg Wildlife Research Institute. Kingsville. Texas. p. 49-73.
5. Briggs, L. J.; Shantz, H. L. 1914. Relative water requirements of plants. Journal of Agricultural Research. 3: 1-63.
6. Britton, N. L.; Rose J. N. 1919. The Cactaceae. Descriptions and Illustrations of plants of the cactus family. Volumes I. New York. Dover Publications. Inc. p. 166. Available online at: http://publicationsonline.carnegiescience.edu/publications_online/cactaceae/ Britton_Rose_Cactaceae_1.pdf (accessed: October 2017).
7. Callaway, R. M. 1995. Positive interactions among plants. The Botanical Review. 61: 306-349.
8. Carrillo-Garcia, A.; Bashan, Y.; Bethlenfalvay, G. J. 2000. Resource-island soils and the survival of the giant cactus, cardon, of Baja California Sur. Plant and Soil. 218: 207-214.
9. Da Silva, J. A.; Bonomo, P.; Donato, S. L. R.; Pires, A. J. V.; Rosa, R. C. C.; Donato, P. E. R. 2012. Composição mineral em cladódios de palma forrageira sob diferentes espaçamentos e adubações química. Revista Brasileira de Ciências Agrárias. 7: 866-875.
10. Donato, P. E. R.; Vieira Pires, A. J.; Rodrigues Donato, S. L.; Da Silva, J. A.; De Aquino, A. A. 2014. Valor nutritivo da palma forrageira 'gigante' cultivada sob diferentes espaçamentos e doses de esterco bovino. Revista Caatinga. 27: 163-172.
11. Dubeux Jr, J. C. B.; Muir, J. P.; Santos, M. V. F.; Cavalcante, M.; dos Santos, D. C. 2013. Potential da palma forrageira na América do sul, in: Nazareno, M. B.; Ochoa, M. J.; Dubeux Jr., J. C. (Eds.). Proceedings of the Second Meeting for the Integral use of cactus pear an other cacti and First South American Meeting of FAO-ICARDA CACTUSNET. Santiago del Estero. Argentina. 29-40.
12. Felker, P. 1995. Forage and fodder production and utilization, in: Barbera, G.; Inglese, P.; Pimienta- Barrios, E. (Eds.), Agro-ecology, cultivation and uses of cactus pear. FAO. Rome. Italy. 144-154.
13. Felker, P.; Paterson, A.; Jenderek, M. M. 2006. Forage potential of Opuntia clones maintained by the USDA. National Plant Germoplasm System (NPGS) collection. Crop Science. 46: 2161-2168.
14. Felker, P.; Zapata, R.; Wang, X; Medina, D.; Bunch, R; Paterson, A. 2010. Fruits characters among apomicts and sexual progeny of a cross of the Texas native Opuntia lindheimerii (1250) with a commercial fruit type Opuntia ficus−indica (1281). Journal of the Professional Association for Cactus Development. 12: 48-66.
15. Fuentes-Rodríguez, J. 1997. A comparison of the nutritional value of Opuntia and Agave plants for ruminants. Journal of the Professional Association for Cactus Development. 2: 20-24.
16. Grassia, J. A. 2009. Zonas climáticas en Argentina. Available online at: http://palmasenresistencia. blogspot.com.ar/2009/09/zonas-climaticas-en-argentina.html (accessed: September 2017).
17. Gregory, R. A.; Felker, P. 1992. Crude protein and phosphorus contents of eight contrasting Opuntia forage clones. Journal of Arid Environments. 22: 323-331.
18. Gregory, R. A.; Kuti, J. O.; Felker, P. 1993. A comparison of Opuntia fruit quality and winter hardiness for use in Texas. Journal of Arid Environments. 24: 37-46.
19. Griffiths, D. 1906. Feeding prickly pear to stock in Texas. U.S. Department of Agriculture, Bureau of Animal Industry. Bulletin N° 91. 44 p.
20. Griffiths, D. 1910. Illustrated Studies in the Genus Opuntia-III. Missouri Botanical Garden Annual Report Vol. 1910. 165-174. Published by: Missouri Botanical Garden Press Stable Available online at: http://www.jstor.org/stable/2400129 Accessed: June 2017 12: 57 UTC).
21. Griffiths, D. 1913. Behavior, under cultural conditions, of species of cacti Known as Opuntia. U. S. Department of Agriculture. 24 p.
22. Griffiths, D. 1915. Hardier spineless cactus: present commercial varieties of prickly pear suited to very limited range-selection of favorable variations in native species gives promise of providing forms that will stand zero temperature. The Journal of Heredity. 6: 182-191.
23. Griffiths, D. 1916. New Species of Opuntia. Bulletin of the Torrey Botanical Club. 43(2): 83-92. Available online at: http://www.jstor.org/stable/pdf/2479824.pdf (accessed: November 2017).
24. Griffiths, D.; Hare, R. F. 1907. Summary of recent investigations of the value of cacti as stock food. U.S. Government Printing Office. 16 p.
25. Griffiths, D.; Hare, R. F. 1907. The tuna as food for man. U.S. Government Printing Office. 73 p.
26. Grünwaldt, J. M.; Guevara. J. C.; Grünwaldt, E. G.; Martínez Carretero. E. 2015. Cacti (Opuntia sps.) as forage in Argentina dry lands. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 47(1): 263-282.
27. Grünwaldt, J. M.; Guevara. J. C.; Martínez Carretero. E.; Grünwaldt, E. G. 2018. Effect of Prosopis spp. as a nurse plant on nutrient content and productivity of Opuntia ellisiana Griffiths. Accepted for publication in Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 50(2): 129-137.
28. Guevara, J. C.; Gonnet, J. M.; Estevez, O. R. 2000. Frost hardiness and production of Opuntia forage clones in the Mendoza plain, Argentina. Journal of Arid Environments. 46: 199-207.
29. Guevara, J. C.; Estevez, O. R. 2002. Opuntia spp. for fodder and forage production in Argentina: experiences and prospects, in: Mondragón-Jacobo, C.; Pérez-González, S. (Eds.), Cactus (Opuntia spp.) as forage. FAO Plant Production and Protection Paper. 169: 63-71.
30. Guevara, J. C.; Silva Colomer, J. H.; Juárez, M. C.; Estevez, O. R. 2003. Opuntia ellisiana: cold hardiness, above-ground biomass production and nutritional quality in the Mendoza plain, Argentina. Journal of the Professional Association for Cactus Development. 5: 55-64.
31. Guevara, J. C.; Felker, P.; Balzarini, M. G.; Paez, S. A.; Paez, M. N.; Antúnez, J. C. 2011. Productivity, cold hardiness and forage quality of spineless progeny of the Opuntia ficus-indica 1281 x O. lindheimerii 1250 cross in Mendoza plain, Argentina. Journal of the Professional Association for Cactus Development. 13: 48-62.
32. Guevara, J. C.; Estevez, O. R. 2018. Sustainable use of rangelands of the Mendoza plain (Argentina). Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 50(1): 295-307.
33. Gutiérrez, J. R.; Meserve, P. L.; Contreras, L. C.; Vásquez, H.; Jaksic, F. M. 1993. Spatial distribution of soil nutrients and ephemeral plants underneath and outside the canopy of Porlieria chilensis shrubs (Zygophyllaceae) in arid coastal Chile. Oecologia. 95: 347-352.
34. Han, H.; Felker, P. 1997. Field validation of water-use efficiency of the CAM plant Opuntia ellisiana in south Texas. Journal of Arid Environments. 36:133-148.
35. Hernández, H. M.; Gómez-Hinostrosa, C.; Bárcenas, R. T.; Puente, R.; Reyes-Agüero, J. A. 2014. A checklist of the subfamily Opuntioideae (Cactaceae) in North and Central America, in: Hunt, D. (Ed.), Further Studies in the Opuntioideae (Cactaceae). The Manse, Chapel Lane, Milborne Port DT9 5DL. England. 185-200.
36. Horno, M. E. 1993. Interceptación de la precipitación por algarrobo, in: IADIZA (Ed.), Contribuciones Mendocinas a la Quinta Reunión Regional para América Latina y el Caribe de la Red de Forestación del CIID. Conservación y mejoramiento de especies del género Prosopis. Mendoza. Argentina. IADIZA. 93-97.
37. Inglese, P.; Liguori, G.; de la Barrera, E. 2017. Ecophysiology and reproductive biology of cultivated cacti, in: Inglese, P.; Mondragon, C.; Nefzaoui, A.; Sáenz, C. (Eds.), Crop ecology, cultivation and uses of cactus pear. FAO. Rome. Italy. 29-41.
38. Juárez, M. C.; Passera, C. B. 2002. In vitro propagation of Opuntia ellisiana Griff. and acclimatization to field conditions. Biocell. 26:319-324.
39. Le Houérou, H. N. 1971. Les Bases Ecologiques de l’amélioration de la Production Fourragère en Algérie. FAO, Rome. Italy. 58 p.
40. Le Houérou, H. N. 1991. Feeding shrubs to sheep in the mediterranean arid zone: intake, performance and feed value, in: Gaston, A.; Kernick, M.; Le Houérou, H. N. (Eds.), Proceedings of the Fourth International Rangeland Congress CIRAD (SCITS) Montepellier. France. 639-644.
41. Le Houérou, H. N. 1994. Drought-tolerant and water-efficient fodder shrubs (DTFS), their role as a "drought insurance" in the agricultural development of arid and semi-arid zones in southern Africa. WRC. Pretoria. South Africa. Report N° KV 65. 139 p.
42. Le Houérou, H. N. 1996a. The role of cacti (Opuntia spp.) in erosion control, land reclamation, rehabilitation and agricultural development in the Mediterranean Basin. Journal of Arid Environments. 33: 135-159.
43. Le Houérou, H. N. 1996b. Utilization of fodder trees and shrubs (TRUBS) in the arid and semiarid zones of western Asia and northern Africa (WANA): history and perspectives. A review. ICARDA/CIHEAM. Hammamet. Tunisia. 51 p.
44. Le Houérou, H. N. 2002. Cacti (Opuntia spp.) as a fodder crop for marginal lands in the Mediterranean basin. Acta Horticulturae. 581: 21-46.
45. Louhaichi, M.; Nefzaoui, A.; Guevara, J. C. 2017. Cactus ecosystem goods and services, in: Inglese, P.; Mondragón, C.; Nefzaoui, A.; Sáenz, C. (Eds.), Crop ecology, cultivation and uses of cactus pear. FAO. ICARDA. Rome. Italy. 159-169.
46. Majure, L. C.; Puente, R.; Pinkava, D. J. 2012. Miscellaneous chromosome numbers in Opuntieae Dc. (Cactaceae) with a compilation of counts for the group. Haseltonia. 18: 67-78.
47. Mares, M. A.; Enders, F. A; Kingsolver, J. M.; Neff, J. L.; Simpson, B. B. 1977. Prosopis as a niche component, in: Simpson, B. B. (Ed.). Mesquite. Its biology in two Desert Scrub Ecosystems. 123-149. U.S./ibp synthesis series 4. Dowden, Hutchinson & Ross, Inc.
48. Mondragón-Jacobo, C.; Pimienta-Barrios, E. 1995. Propagation. in: Barbera, G.; Inglese, P.; Pimienta-Barrios, E. (Eds.), Agro-ecology, cultivation and uses of cactus pear. Rome. Italy. FAO. 64-70.
49. Mondragón Jacobo, C.; Pérez González, S. 2001. Germplasm resources and breeding opuntia for fodder production, in: Mondragón Jacobo, C.; Pérez González, S. (Eds.), Cactus (Opuntia spp.) as forage. FAO. Rome. Italy. p. 21-28.
50. Monjauze, A.; Le Houérou, H. N. 1965. Le rôle des Opuntia dans l'Economie agricole Nord Africaine. Extrait du Bulletin de l'Ecole Nationale Supérieure d'Agriculture de Tunis. 8-9: 85-164.
51.Nobel, P. S. 1988. Environmental biology of agaves and cacti. New York. USA: Cambridge University Press. 270 p.
52. Nobel, P. S. 1991. Achievable productivities of CAM plants: basis for high values compared with C3 and C4 plants. Tansley Review 32. New Phytologist. 119: 183-205.
53. Nobel, P. S. 1994. Remarkable agaves and cacti. Oxford University Press, New York. 180 p.
54. Nobel, P. S. 1995. Environmental biology, in: Barbera, G.; Inglese, P.; Pimienta-Barrios, E. (Eds.), Agroecology, cultivation and uses of cactus pear. FAO Plant Production and Protection Paper N° 132. 36-48.
55. Nobel, P. S. 2001. Ecophysiology of Opuntia-ficus-indica, i n: Mondragón-Jacobo, C.; Pérez- González, S. (Eds.), Cactus (Opuntia spp.) as forage. FAO Plant Production and Protection Paper. 169: 13-20.
56. Nobel, P. S. 2002. Cactus physiological ecology, emphasizing gas exchange of Platyopuntia fruits. Acta Horticulturae. 581: 143-150.
57. Nobel, P. S.; Bobich, E. G. 2002. Environmental Biology, in: Nobel, P. S. (Ed.), Cacti: biology and uses. University of California Press. Berkeley. CA. USA. 57-74.
58. Nobel, P. S.; Hartsock, T. L. 1984. Physiological response of Opuntia ficus-indica to growth temperature. Physiologia Plantarum. 60: 90-105.
59. Parish, J.; Felker, P. 1997. Fruit quality and production of cactus pear (Opuntia spp.) fruit clones selected for increased frost hardiness. Journal of Arid Environments. 37: 123-143.
60. Pimienta B., E. 1990. El Nopal Tunero. Departamento de Investigación Científica y Superación Académica de la Universidad de Guadalajara. UG. Guadalajara Jalisco. México. 246 p.
61. Pimienta, E. 2002. Preface, in: Mondragón-Jacobo, C.; Pérez-González, S. (Eds.), Cactus (Opuntia spp.) as forage. FAO Plant Production and Protection. Paper 169.
62. Powell, A. M.; Weedin, J. F. 2001. Chromosome numbers in Chihuahuan Desert Cactaceae. III. Trans Pecos Texas. American Journal of Botany. 88: 481-485.
63. Pugnaire, F. I.; Haase, P.; Puigdefabregas, J. 1996. Facilitation between higher plant species in a semiarid environment. Ecology. 77: 1420-1426.
64. Russell, C. E.; Felker, P. 1987. The prickly pears (Opuntia spp., Cactaceae): a source of human and animal food in semiarid regions. Economic Botany. 41: 433-445.
65. Scalisi, A.; Morandi, B.; Inglese, P.; Lo Bianco, R. 2015. Cladode growth dynamics in Opuntia ficus-indica under drought. Environmental Experimental Botany. 122: 158-167.
66. Sinclair, T. R.; Tanner, C. B.; Bennett, J. M. 1984. Water-use efficiency in crop production. Bioscience. 34: 36-40.
67. Suzán-Azpiri, H.; Sosa, V. J. 2006. Comparative performance of the giant cardon cactus (Pachycereus pringlei) seedlings under two leguminous nurse plant species. Journal of Arid Environments. 65: 351-362.
68. Ting, I. P. 1985. Crassulacean acid metabolism. Annual Review of Plant Physiology. 36: 595-622.
69. USDA, NRCS. 2012. Available online at http://planthardiness.ars.usda.gov/PHZMWeb/ (accessed: September 2017).
70. Wang, X.; Felker, P.; Paterson, A. 1997. Environmental influences on cactus pear fruit yield, quality and cold hardiness and development of hybrids with improved cold hardiness. Journal of the Professional Association for Cactus Development. 2: 48-59.
71. Weedin, J. F.; Powell, A. M. 1978. Chromosome numbers in Chihuahuan desert Cactaceae. Trans-Pecos Texas. American Journal of Botany. 65: 531-537.
72. Winter, K. 1985. Crassulacean acid metabolism, in: Barber, J.; Baker, N. R. (Eds.), Photosynthetic mechanisms and environment. Elsevier. Amsterdam: 329-387.
73. Winter, K.; Smith, J. A. C. 1996. An introduction to Crassulacean Acid Metabolism. Biochemical Principles and Ecology Diversity, in: Winter, K.; Smith, J.; Andrew, C. (Eds.), Crassulacean Acid Metabolism. Biochemistry, Ecophysiology and Evolution. Springer-Verlag Berlin Heidelberg: 1-13.