Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 55(2). ISSN (en línea) 1853-8665.
Año 2023.
Original article
Susceptibility
of Rhyzopertha dominica (Coleoptera: Bostrichidae) and Sitophilus
oryzae (Coleoptera: Curculionidae) to the fungal entomopathogen Beauveria
bassiana (Balsamo-Crivelli) Vuillemin s.l. (Hypocreales: Clavicipitaceae)
Susceptibilidad
de Rhyzopertha dominica (Coleoptera: Bostrichidae) y Sitophilus
oryzae (Coleoptera: Curculionidae) al hongo entomopatógeno Beauveria
bassiana (Balsamo-Crivelli) Vuillemin s.l. (Hypocreales: Clavicipitaceae)
1Universidad
Nacional de La Plata. Facultad de Ciencias Naturales y Museo. 122 y 60.
Instituto Spegazzini. S/n La Plata 1900. Buenos Aires. Argentina.
2Comisión
de Investigaciones Científicas de la Provincia de Buenos Aires CICPBA.
3Consejo
Nacional de Investigaciones Científicas y Técnicas (CONICET).
*russomaleticia@gmail.com
Abstract
Control measures
of stored grain pests include the excessive utilization of chemical
insecticides that generate negative environmental impact. Current trends in
integrated pest management are oriented towards the preservation of the
environment using natural biopesticides, among these products arise
entomopathogenic fungi. This study aimed to test the efficacy of a native
strain of Beauveria bassiana to control two main stored grain pests such
as Sitophilus oryzae and Rhyzopertha dominica and also evaluate
the persistence of the fungus on wheat grains. The B. bassiana strain
controlled 89%±0.07 of R. dominica adults and 80% ±0.14 of S. oryzae.
The survival analysis showed that MST was 4.27 ±0.19 days for R.
dominica adults and 4.27 ±0.20 days for S. oryzae. Furthermore,
results of long Rank test for the comparison of the Kaplan-Meier curves did not
present significant differences between the survival of both stored grain
pests. Dual choice tests demonstrated that B. bassiana LPSc1227
presented a repellent action against both stored grain pests. The seed
persistence of conidia was 100% in treated seeds after 45 days. Further
research will contribute to elucidate more insecticidal features of the B.
bassiana LPSc 1227 strain against S. oryzae and R. dominica,
two main stored grain insect pests.
Keywords: stored grain
pests, fungal entomopathogens, biopesticides
Resumen
Las medidas de
control de plagas de granos almacenados incluyen la utilización excesiva de
insecticidas químicos generando un impacto negativo al ambiente. Las tendencias
actuales en el manejo integrado de plagas están orientadas a la preservación
del ambiente utilizando biopesticidas naturales, entre estos surgen los hongos
entomopatógenos. Este estudio tuvo como objetivo probar la eficacia de una cepa
nativa de Beauveria bassiana para controlar dos de las principales
plagas de granos almacenados, Sitophilus oryzae y Rhyzopertha
dominica, y también evaluar la persistencia del hongo en granos de trigo.
La cepa B. bassiana controló el 89% de los adultos de R. dominica y
el 80% de S. oryzae. El análisis de supervivencia mostró que el TMS fue
de 4,27 días para R. dominica y de 4,27 días para S. oryzae.
Además, los resultados del “long rank” test no presentaron diferencias
significativas en la supervivencia de ambas plagas. La prueba de elección
demostró que B. bassiana presentó una acción repelente frente a ambas
especies de insectos. La persistencia de los conidios en las semillas fue del
100% en las semillas tratadas. Futuros estudios permitirán dilucidar la
capacidad de B. bassiana LPSc 1227 para controlar los principales
insectos plaga de granos almacenados, S. oryzae y R. dominica.
Palabras clave: plagas de granos
almacenados, entomopatógenos fúngicos, biopesticidas
Originales: Recepción: 30/08/2023 - Aceptación: 02/11/2023
Introduction
Food production
faces the challenge of keeping up high levels of quality, considering aspects
of food safety and production systems with fair remuneration for producers (8). Grain storage arises as a consequence of the
randomness and seasonality of agricultural production. Inside stored
commodities, the temperature and humidity conditions favor the appearance of
insect pests that find the food and protection to display their multiplication
potential. It has been estimated that 5-30% of post-harvested losses worldwide
are due to insect damage (25). The
damages are qualitative and quantitative, including reduction of hectoliter
weight, increased commercial rejection levels, alterations in the nutritional
value, deterioration of industrial features and decreased seed germination
power (13). Some species in the Order
Coleoptera, due to their ubiquity and high destructive potential, constitute
one of the greatest entomological problems of stored grains. The “weevil” (Sithophilus
oryzae L.) and the “cereal borer” (Rhyzopertha dominica F.) are
primary infestation beetles that initiate the deterioration of healthy grains,
the larvae feed on the endosperm, leaving holes that facilitate the entry of
secondary infestation species (13).
Despite the
negative consequences associated with the utilization of synthetic
insecticides, these substances are still the main solution utilized in
preventive and curative treatments of stored grains. The use of
chemical-synthetic insecticides involves a series of disadvantages: such as the
presence of toxic residues in the grain, intoxication of users and consumers,
contamination of the environment, and development of insect resistance (1, 4). The urgent need for a control method that
ensures the elimination of insect pests, leads in many cases to incorrect and
excessive applications of these harmful products, risking food safety and
therefore the health of the consumer, generating a negative environmental
impact and the rejection of grains in the market (13).
The current
demand for healthier food and the change in production paradigms require the
total or partial replacement of synthetic pesticides by non-polluting methods.
In this sense, a biological alternative, not detrimental to the environment
that is also safe for the producers and the consumers is urgently needed to
control stored grain pests. Current trends in integrated pest management are
oriented towards preserving the environment together with the use of natural
biopesticides with less toxicity. Among these products are the entomopathogenic
fungi (14, 20).
The species
within the genera Beauveria and Metarhizium are widely used due
to their specificity and effectiveness as biological insecticides (11, 22, 26, 27). Several studies have
demonstrated the capacity of entomopathogenic fungi to protect stored seeds and
have demonstrated their insecticidal capacity to control different beetles (3, 9, 10, 13, 14) highlighting the importance of
conducting bioassays for the selection of highly virulent isolates given the
high genetic variability presented by these microorganisms.
The hypotheses
tested were that native strains are able to control several species of stored
grain pests and that the fungus is able to persist on the surface of wheat
grains. Thus, this study was conducted to test the efficacy of a native strain
of B. bassiana to control two main stored grain pests such as S.
oryzae and R. dominica and also evaluate the persistence of the
fungus on wheat grains in laboratory conditions.
Material
and methods
Insect
rearing
Two main stored
grain pests R. dominica and S. oryzae, were selected to perform bioassays.
The insects were acquired from the Department of Agricultural Zoology (Faculty of
Agronomy, National University of Buenos Aires, Argentina) and a laboratory
colony was established at the Spegazzini Institute, La Plata National University,
Argentina. The insects were kept inside glass containers (400 mL) and provided
with wheat grains (cultivar Klein Capricornio, Cauda Semillas, Chacabuco,
Argentina) as food source. The colonies were maintained in a climatic chamber
under controlled conditions of temperature and humidity (26 ±2°C and 70±5% RH).
All trials were
carried out with adults of 7-10 days of age.
Fungal
strain
The fungal
strain used to carry out the laboratory tests was provided by the mycological collection
of the Spegazzini Institute, La Plata National University, Argentina. The Beauveria
bassiana LPSc 1227 strain (GeneBank Accession number MG012792) was isolated
from Schistocerca cancellata (Orthoptera: Acrididae) in Santiago del
Estero Province, Argentina during 2016. This strain was selected based on its
entomocidal capacity (15). The inocula was obtained from cultures
maintained in potato dextrose agar (PDA) for a week at 25°C in the dark.
Conidia were harvested with a sterile loop, placed in test tubes and stirred
for 2 minutes using a vortex. The conidial concentration was determined as in Goettel & Inglis (1997). The conidia were counted
using a Neubauer chamber under a light microscope and the concentration was
adjusted to 1×108 conidia/ml. The viability of the conidia was determined
according to Goettel & Inglis (1997). The fungal
suspensión (400 μl) was inoculated into slides containing a thin layer of PDA
culture media. Slides were kept for 24 h in Petri dishes containing a moistened
filter paper to allow conidia germination. Conidia were considered germinated
when the germ tube exceeded half its length. Three repetitions were made at
different times and 300 conidia were counted on each case. The conidia exhibited
a 99% germination rate.
Mortality
test
Rhyzopertha
dominica and
S. oryzae adults were inoculated with the fungal solution (1×108
conidia/ml) using a hand glass sprayer (20 mL). Control insects were sprayed with
a conidia free solution of Tween 80 ® (Merck) 0.01% (v/v). Afterwards, adults
were individualized in Petri dishes containing wheat grain as food source and
placed inside a climatic chamber under controlled conditions (24±2°C, 75%).
Mortality was recorded daily for 14 days. Humid chambers were set up according
to Goettel & Inglis (1997) to confirm death by
mycosis.
As data did not
meet normality insect mortality was analyzed using the Wilcoxon test.
Survival curves
and mean survival time (MST) were estimated using the Kaplan-Meyer analysis.
Pairwise comparisons between survival curves were made by Logrank test (6, 15). Infostat software was used to performe
the statistical analyses (5).
Choice
test
A dual-choice
olfactometer with static air (supplementary material) was utilized to evaluate
the insect preference according to Mitina et al. (2020)
methods. One pot (20 cm3) containing 20 g of treated wheat grains was placed at
one end of the tube and a container with untreated grains (control) was placed
at the other end. Treated group grains were sprayed with the conidial
suspension (1× 108conidia/ml). In the control group, grains were sprayed with a
Tween 80 solution. Insects were placed individually at the center of the
olfactometer, and the chosen direction was recorded. The olfactometer was
rotated to ensure that the behavior of the insects depended only on the
repellent action. Individuals who did not show a response after 10 minutes were
not considered in the analysis. A total of 100 insects of each species were
employed in this experiment.
To characterize
the olfactory response of weevils, the “index of aggregation” (19, 29) was calculated using the following
formula:
IA= (O-K)/ (O+K)
× 100%
where:
O = number of
insects in the tube with the treated sample
K = number of
insects in the control tube
If differences
are obtained between the mean number of insects in the treatment and control,
and the value of the index is positive indicates an attractive effect of the
sample. On the other hand, a repellent action of the sample is recorded when
the value of the index is a negative number. If no differences are observed a
neutral action of the sample is inferred (19, 29).
Seed
persistence
To evaluate the
conidial persistence in wheat over time, seeds (100 g) were autoclaved for 20
minutes, and then were sprayed with 30 ml of the fungal suspension
(1×108conidia/ml). Inoculated seeds were placed in Erlenmeyer flasks (250 ml)
and were maintained at 24°C in the darkness. Three seeds were randomly selected
every week for 45 days and were placed in Petri dishes containing PDA. Control
seeds were not mixed with the fungal inocula. The Petri dishes were kept under
controlled conditions of temperature (25°C) and in darkness for seven days. The
persistence was recorded when fungal colonies grew around seeds.
Results
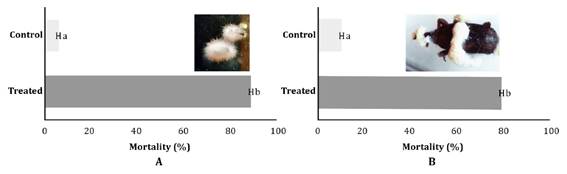
Mortality tests
showed significant differences between treatments for both insect species, R.
dominica (W=135, p < 0.0001) and S. oryzae (W=55, p=0.0001). The B.
bassiana strain controlled 89%±0.073 of R. dominica adults and 80%
±0.14 of S. oryzae (figure 1).
Different letters show significant differences
according to Wilcoxon test (<0.05).
Letras diferentes indican diferencias significativas
de acuerdo con el test de Wilcoxon (<0,05).
Figure 1.
Percentage of adult mortality after the treatment with Beauveria bassiana LPSc
1227 strain. A: Rhyzopertha dominica and B: Sitophilus
oryzae.
Figura 1. Mortalidad
porcentual de adultos de A: Rhyzopertha dominica y B: Sitophilus
oryzae inoculados con la cepa de Beauveria bassiana LPSc 1227.
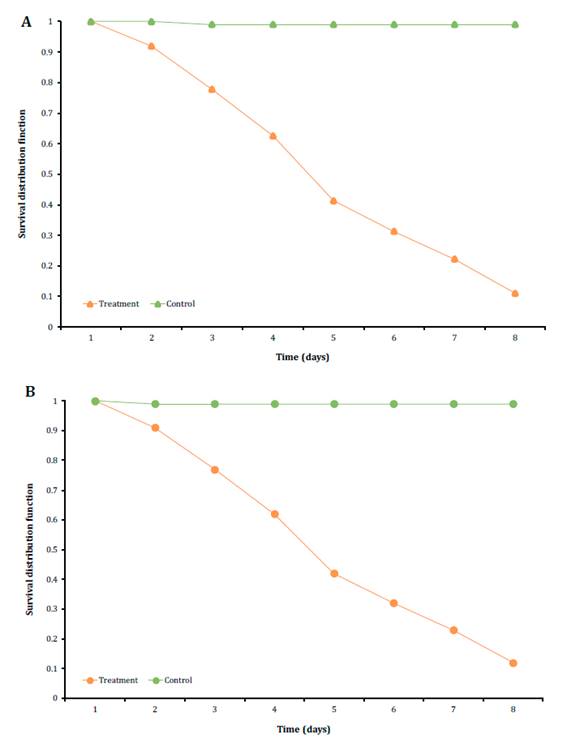
The Kaplan-Meier
analysis exhibited a mean survival time (MST) of 4.27 ±0.19 days for R.
dominica adults and 4.27 ±0.20 days for S. oryzae. Results of long
rank test (X2) for the comparison of the Kaplan-Meier curves did not present
significant differences between the survival of both stored grain pests
(log-rank test p>0.05) (figure 2).
Figure 2.
Kaplan-Meier survival curves of Rhyzopertha dominica A: and Sitophilus
oryzae B: after the treatment with Beauveria bassiana LPSc
1227 strain.
Figura 2. Curvas
de supervivencia de Kaplan-Meier A: Rhyzopertha dominica y B:
Sitophilus oryzae luego del tratamiento con la cepa de Beauveria
bassiana LPSc 1227.
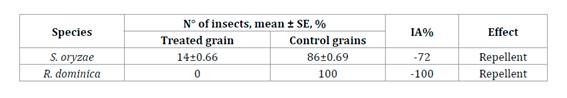
Dual
choice tests demonstrated that B. bassiana LPSc1227 presented a
repellent action against both stored grain pests (table 1).
Table
1. Effect of Beauveria bassiana on
treated wheat grains on the preference of Sitophilus oryzae and Rhizopertha
dominica.
Tabla 1. Efecto
de Beauveria bassiana sobre la preferencia por granos tratados de Sitophilus
oryzae y Rhizopertha dominica para granos de trigo con y sin
tratamiento de Beauveria bassiana.
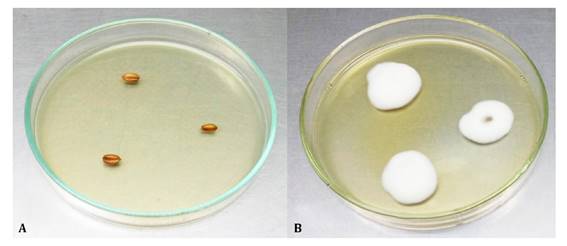
After 45 days,
100% of wheat seeds studied preserved viable conidia on the surface (figure 3).
A: Control
(not inoculated) and B: Treated with Beauveria bassiana LPSc
1227.
A: Control
(no inoculadas) y B: Tratadas con Beauveria bassiana LPSc 1227.
Figure 3:
Persistence of Beauveria bassiana conidia 45 days after the wheat grain
treatment.
Figura 3: Persistencia
de conidios de Beauveria bassiana en semillas de trigo luego de 45 días.
The results
provided by this investigation constitute a starting point in the utilization
of the B. bassiana strain LPSc 1227 as an effective entomopathogen to
control two main stored grain pests.
In this study, B.
bassiana LPSc 1227 exhibited mortality levels of 80-89% against R.
dominica and S. oryzae respectively, showing a good performance to
control both coleopteran species in vitro. Many studies presented
similar results where the effectiveness of the entomopathogens relied on the
fungal strain and the pest species tested. Wakil et
al. (2021 b) found that R. dominica was the most susceptible
species to B. bassiana and M. anisopliae. Also, Reza Pourian & Alizadeh (2021) reported that an
isolate of B. bassiana was effective in killing 60-73% of Callosobruchus
maculatus (F.) (Chrysomelidae) and Oryzaephilus surinamensis (L.) (Silvanidae).
Kordali et al. (2021) registered high
mortalities (from 62.6% to 100%) of C. maculatus adults using different
species of entomopathogenic fungi. Furthermore, Yanar et
al. (2019) also found that the S. granarius mortality varied
depending on the fungal isolate utilized registering up to 70% mortalities.
When analyzing fungal entomopathogens against R. dominica, Musso et al. (2020) reported that B. bassiana
strains were the most effective in controlling adults causing up to 65%
mortality.
Mean survival
time (MST) constitutes an important parameter to describe when characterizing
entomopathogens that gives an approximation of the pathogenicity rate of the
fungus. In this study, median lethal times were similar for both insects
(4.27±0.19 for R. dominica and 4.27±0.20 for S. oryzae)
confirming the good performance of the fungus.
In the Same
fashion, El Khourry et al. (2022) estimated
for four different stored grain pests MST of 3.5±0.3 for Cathartus
quadricollis (Guerin-Meneville) (Coleoptera: Silvanidae), of 3.8±0.6 days
for Callobrosuchus maculatus F. (Coleoptera: Chrysomelidae), of 3.8±0.2
for Sitophilus granarius L. (Coleoptera: Curculionidae) and of 4.1±0.2
for Oryzaephilus surinamensis L. (Coleoptera: Silvanidae) using a B.
bassiana strain. Similarly, Kassa et al. (2002)
when studying several strains of B. bassiana to control Sitophilus
zeamais Motschulsky (Coleoptera: Curculionidae) and Protephonus
truncates Horn (Coleoptera: Bostrichidae) registered MST that ranged
between 2.85±0.05 to 6.28±0.41 days. On the contrary, higher MST have been
found by other authors indicating a poor entomodicidal capacity of the strains
employed, for instance Al-Zunti et al. (2023)
when studying the effect of B. bassiana on larval stages of Tribolium
castaneum Herbst (Coleoptera: Tenebrionidae) found MST of 5-6 days and in
the case of R. dominica, Musso et al. (2020)
obtained MST values for B. bassiana strains of 8 to 9 days.
The differential
susceptibility of different species of stored grain insect pests towards fungal
entomopathogens has been attributed to differences in the composition of the
insect cuticle, to the conidial concentration or to fungal specificity (3, 4, 27). The entomocidal capacity of the B.
bassiana LPSc 1227 strain towards two of the main primary stored grain
pests represents an important finding in the search for the formulation of an
effective bioinsecticide.
Fungal-insect
interactions are of great interest to understand the fundamental behavioral
processes that occur between insects and pathogens. This issue remains crucial
when trying to exploit fungal entomopathogens as biological control agents.
Results provided by this study show that the B. bassiana strain
inflicted a repellent effect on both insect species. Many studies revealed that
insects tend to avoid the presence of fungal entomopathogens (16, 17, 23). This behavior has been attributed to
volatile compounds released by fungi and to the capacity of insects to detect
these specific signals. In this regard, Selitskaya et
al. (2016) confirmed that B. bassiana strain Yuk-4 had a strong
repellent effect towards the granary weevil. Similar results were also obtained
by Mitina et al. (2020) using B. bassiana.
The authors obtained negative values of the index of aggregation, showing repellence
towards the fungus. Also, Selitskaya et al. (2014)
investigated the behavior response of S. oryzae to several Fusarium strains
and found differential responses of the insects according to the strains.
Seed persistence
of conidia on wheat grains over prolonged periods may provide extra protection
against stored primary pests since it may contribute to suppressing progeny
production (27). Results in this study
demonstrated that the strain B. bassiana LPSc 1227 remains viable on the
seed surface for at least 45 days.
When developing
a microorganism-based product the study of multitrophic interactions should be
considered and included as part of the basic research of an entomopathogen,
future research will contribute to elucidating further properties of the
promising strain studied.
Conclusion
This study
demonstrates that the native strain of B. bassiana LPSc 1227 can
effectively control stored grain pests and also persist on the surface of wheat
grains.
Further research
will contribute to elucidating additional insecticidal features of the B.
bassiana LPSc 1227 strain against S. oryzae and R. dominica,
two primary pests of stored grain. Additionally, it aims to evaluate whether
the conidia present on the seed surface after 45 days retain their germination
and insecticidal capacity.
1. Agrafioti,
P.; Athanassiou, C. G. G. 2018. Insecticidal effect of contact insecticides
against stored product beetle populations with different susceptibility to
phosphine. Journal of Stored Product Research. 79: 9-15.
https://doi.org/10.1016/j.jspr.2018.06.002
2. Al-Zunti, S.;
Kareem, A. A.; Alamry, A. T.; Kadhem, Z. J.; Port, G.; Sanderson, R. 2023. The
Efficiency of Beauveria bassiana, Metarhizium anisopliae and Lecanicillium
muscarium against different stages of the flour beetle, Tribolium castaneum
(Herbst) (Coleoptera: Tenebrionidae). Journal of Kerbala for Agricultural
Sciences. 10(2): 15-32. https://doi.org/10.59658/jkas.v10i2.1182
3. Barra, P.;
Rosso, L.; Nesci, A.; Etcheverry, M. 2013. Isolation and identification of
entomopathogenic fungi and their evaluation against Tribolium confusum, Sitophilus
zeamais, and Rhyzopertha dominica in stored maize. Journal of Pest
Science. 86: 217-226. https://doi.org/10.1007/s10340-012-0460-z
4. Batta, Y. A.;
Kavallieratos, N. G. 2018. The use of entomopathogenic fungi for the control of
stored-grain insects, International Journal of Pest Management. 64(1): 77-87.
https://doi.org/10.1080/09670874.2017.1329565
5. Di Rienzo, J.
A.; Casanoves, F.; Balzarini, M. G.; González, L.; Tablada Robledo, C. W. 2011
Grupo InfoStat. FCA. Universidad Nacional de Córdoba. Argentina.
http://www.infostat.com.ar
6. El Khoury,
Y.; Bari, G.; Salvemini, C.; Altieri, G.; Karimi, J.; Poliseno, M.; Tarasco, E.
2022. Susceptibility of four stored-product insect pests to Beauveria
bassiana and Metarhizium anisopliae strains. Redia: Giornale di
Zoologia. 105: 175-182 http://dx.doi. org/10.19263/REDIA-105.22.22
7. Goettel, M.
S.; Inglis, G. D. 1997. Fungi: hyphomycetes. In: Lacey LA (ed) Manual of
techniques in insect pathology. Academic Press. San Diego. 231-248.
8. Gutiérrez, C.
G.; Maldonado, M. G. 2010. Uso de bioinsecticidas para el control de plagas de
hortalizas en comunidades rurales. Ra Ximhai: revista científica de sociedad,
cultura y desarrollo sostenible. 6(1): 17-22.
https://doi.org/10.1016/j.jip.2019.107254
9. Iqbal, J.;
Ahmad, S.; Ali, Q. 2021. A comparative study on the virulence of
entomopathogenic fungi against Trogoderma granarium (Everts)
(Coleoptera: Dermestidae) in stored grains rice. Brazilian Journal of Biology.
82. https://doi.org/10.1590/1519-6984.250778
10. Kassa, A.;
Zimmermann, G.; Stephan, D.; Vidal, S. 2002. Susceptibility of Sitophilus
zeamais (Motsch.) (Coleoptera: Curculionidae) and Prostephanus truncatus
(Horn) (Coleoptera: Bostrichidae) to Entomopathogenic Fungi from Ethiopia,
Biocontrol Science and Technology. 12(6): 727-736. DOI:
10.1080/0958315021000039905
11. Khoobdel,
M.; Pourian, H. R.; Alizadeh, M. 2019. Bio-efficacy of the indigenous entomopathogenic
fungus, Beauveria bassiana in conjunction with desiccant dust to control
of coleopteran stored product pests. Journal of Invertebrate Pathology. 168:
107254. https://doi.org/10.1016/j.jip.2019.107254
12. Kordali, Ş.;
Bozhuyuk, A. U.; Kesdek, M.; Altinok, H.; Altinok, M. A. 2021. Efficacy of
various entomopathogenic fungi strains as biocontrol agents for control of Callosobruchus
maculatus (Fabricius) (Coleoptera: Bruchidae). Journal of Agricultural
Sciences. 27(4): 454-459. https://doi.org/10.15832/ankutbd.702271
13. Kumar, R.
2017. Insect pests of stored grain: Biology, behavior, and management
strategies. CRC Press.
14. Mantzoukas,
S.; Lagogiannis, I.; Kitsiou, F.; Eliopoulos, P. A. 2023. Entomopathogenic
Action of Wild Fungal Strains against Stored Product Beetle Pests. Insects. 14:
91. https://doi.org/10.3390/insects14010091
15. Mariottini,
Y.; Lange, C. E.; Pelizza, S. E. 2022. Laboratory test of Beauveria bassiana
(Balsamo-Crivelli) Vuillemin sl (Hypocreales: Clavicipitaceae) baits for the
biocontrol of the Toad grasshopper pest, Bufonacris claraziana (Saussure)
(Orthoptera: Tristiridae). Egyptian Journal of Biological Pest Control. 32(1):
110. https://doi.org/10.1186/s41938-022-00609-4
16. Meyling, N.
V.; Pell, J. K. 2006. Detection and avoidance of an entomopathogenic fungus by
a generalist insect predator. Ecological Entomology. 31(2): 162-171.
https://doi.org/10.1111/j.0307-6946.2006.00781.x
17. Mitina, G.
V.; Selitskaya, O. G.; Schenikova, A. V. 2020. Effect of Volatile Compounds of the
Entomopathogenic Fungi Beauveria bassiana (Bals.-Criv.) Vuill. and Lecanicillium muscarium R. Zare et W. Gams on the
Behavior of Sitophilus granarius (L.) (Coleoptera, Dryophthoridae) and
Evaluation of the Virulence of Different Strains of These Fungi. Entmol. Rev.
100: 456-462. https://doi.org/10.1134/S001387382004003X
18. Musso, A.;
Marcondes Almeida, J. E.; Padín, S. B.; Ordoqui, E.; Lopez Lastra, C. C. 2020.
Efficacy of entomopathogenic fungi against Rhyzopertha dominica (Fabricius)
(Coleoptera: Bostrichidae) under laboratory conditions. Revista de la Facultad
de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 52(2):
317-324.
19.
Pascual-Villalobos, M. J.; Robledo, A. 1999. Anti-insect activity of plant
extracts from the wild flora in southern Spain. Biochemical Systematics and
Ecology. 27(1): 1-10. https://doi.org/10.1016/S0305-1978(98)00051-9
20. Pelizza, S.;
Mancini, M.; Russo, L.; Vianna, F.; Scorsetti, A. C. 2023. Capacidad de control
de la cepa LPSc 1067de Beauveria bassiana (Ascomycota: Hypocreales)
sobre diferentes especies de tucuras (Orthoptera: Acrididae: Melanoplinae),
plagas del agro de Argentina. Revista
de la Facultad de Ciencias Agrarias . Universidad Nacional de Cuyo.
Mendoza. Argentina. 55(1): 98-103 DOI: https://doi.org/10.48162/rev.39.099.
21. Reza
Pourian, H.; Alizadeh, M. 2021. Diatomaceous earth low-lethal dose effects on
the fitness of entomopathogenic fungus, Beauveria bassiana, against two
coleopteran stored product pests. Journal of Stored Products Research. 94:
101878. https://doi.org/10.1016/j.jspr.2021.101878
22. Rumbos, C.
I.; Athanassiou, C. G. 2017. Use of entomopathogenic fungi for the control of stored-product
insects: can fungi protect durable commodities? Journal of Pest Science .
90: 839-854. https://doi.org/10.1007/s10340-017-0849-9
23. Selitskaya,
O. G.; Gavrilova, O. P.; Schenikova, A. V.; Shamshev, I. V.; Gagkaeva, T. Y.
2014. The effect of toxin-producing Fusarium fungi on behavior of the
rice weevil Sitophilus oryzae (Coleoptera, Dryophthoridae).
Entomological review. 94: 820-825. https://doi.org/10.1134/S0013873814060037
24. Selitskaya,
O. G.; Mitina, G. V.; Schenikova, A. V.; Choglokova, A. A.; Levchenko, M. V.
2016. Effects of volatiles of entomopathogenic fungi on behavioral responses of
storage pests. Vestn. Zashch. Rast. 89: 3.
25. Singh, K.
D.; Mobolade, A. J.; Bharali, R.; Sahoo, D.; Rajashekar, Y. 2021. Main plant
volatiles as stored grain pest management approach: A review. Journal of
Agriculture and Food Research. 4: 100127.
https://doi.org/10.1016/j.jafr.2021.100127
26. Wakil, W.;
Schmitt, T.; Kavallieratos, N. G. 2021(a). Mortality and progeny production of
four stored-product insect species on three grain commodities treated with Beauveria
bassiana and diatomaceous earths. Journal of Stored Products Research. 93:
101738. https://doi.org/10.1016/j.jspr.2020.101738
27. Wakil, W.;
Kavallieratos, N. G.; Ghazanfar, M. U.; Usman, M.; Habib, A.; El-Shafie, H. A.
2021(b). Efficacy of different entomopathogenic fungal isolates against four
key stored-grain beetle species. Journal of Stored Products Research. 93:
101845. https://doi.org/10.1016/j.jspr.2021.101845
28. Yanar, Y.;
Yanar, D.; Demir, B.; Karan, Y. B. 2019. Effects of local entomopathogenic Beauveria
bassiana isolates against Sitophilus granarius (Coleoptera).
Poljoprivreda i Sumarstvo. 65(1): 49-55. https://doi.org/
10.17707/AgricultForest.65.1.05
29. Zakladnoy,
G. A. 1983. Zashchita zerna i produktov ego pererabotki ot vreditelei
(Protection of Grain and Grain Products from Pests), Moscow: Kolos.
Supplemmentary
material
Squematic figure of the
dual-choice olfatometer utilized in choice test with stored grain pests: https://drive.google.com/file/d/1dRT3o9FaT9fXJbjrz71VPs3ybds2wukv/view?usp=sharing
Funding
This study was
partially supported by the Agencia Nacional de Promoción Científica y
Tecnológica (PICT 2019-1569; PICT Start Up 2020-0008, PICT 2021-0127, PICT
2021-0347) and Universidad Nacional de La Plata (UNLP, 11/N 903).