Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(1). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Green
solvents for the recovery of phenolic compounds from strawberry (Fragaria x ananassa Duch) and apple (Malus
domestica) agro-industrial bio-wastes
Uso
de solventes verdes para la extracción de compuestos fenólicos a partir de
residuos agroindustriales de frutilla (Fragaria
x ananassa Duch) y manzana (Malus domestica)
1Universidad
Nacional del Litoral. Facultad de Ingeniería Química. Instituto de Tecnología
de Alimentos. Santiago del Estero 2829. 3000. Santa Fe. Argentina.
2Consejo
Nacional de Investigaciones Científicas y Técnicas (CONICET). 3000. Santa Fe.
Argentina.
*ampiagen@fiq.unl.edu.ar
Abstract
We aimed to
study the obtention of valuable phenolic compounds from tissue by-products of
agro-industrial processing of apple (GS) and strawberry (RF) using green
solvents and Soxhlet extraction methodology. The effects of solvent type [water
(W); 80% ethanol, (EtOH)] and extraction ratio (1:10, 1:20, 1:30, and 1:40 p/v)
were determined on total phenolic content (TPC), antioxidant capacity (DPPH),
and the profile of phenolic compounds of the GS and RF extracts. The solvent
type and the extraction ratio significantly affected TPC and DPPH of GS and RF
extracts. Extraction with EtOH and 1:40 ratio produced the highest yields,
obtaining an RF extract with 15.8 g GAE/Kg (TPC) and 19 mmol TE/Kg (DPPH). The
tetra-galloyl glucose isomer and agrimoniin (0.8-0.4 g/Kg) were the main RF
phenolic compounds of the eight identified. GS extract, obtained with EtOH and
1:40 ratio, had 11.9 g GAE/Kg (TPC) and 20.5 mmol TE/Kg (DPPH), having
quercetin -3-o-glucuronide (0.43 g/Kg) the highest concentration among the
eight phenolic compounds identified. The results highlight the potential of
green solvents to obtain valuable compounds from low-cost raw materials, like
the high-antioxidant capacity phenolic compound extracts obtained herein.
Keywords: green
extraction, bioactive compounds, hydrolysable tannins, flavonols, natural
antioxidants
Resumen
El objetivo fue
estudiar la extracción de compuestos fenólicos con alto valor bioactivo, a
partir de subproductos del procesamiento de manzana (GS) y frutilla (RF),
utilizando solventes amigables con el medio ambiente y la extracción Soxhlet.
Se determinaron los efectos del tipo de solvente [agua (W); etanol 80% (EtOH)]
y la relación de extracción (1:10, 1:20, 1:30 y 1:40 p/v) sobre el contenido de
fenoles totales (TPC), capacidad antioxidante (DPPH) y perfil de compuestos
fenólicos. El tipo de solvente y la relación de extracción afectaron
significativamente el TPC y la DPPH. La extracción con EtOH a 1:40 produjo los
rendimientos más altos, obteniendo un extracto de RF con 15.8 g GAE/Kg (TPC) y
19 mmol TE/Kg (DPPH). El isómero de tetra galoil glucosa y agrimoniin (0,8-0.4
g/Kg) fueron los principales compuestos fenólicos de RF, entre los ocho
identificados. El extracto de GS, obtenido con EtOH a 1:40, tuvo 11,9 g GAE/Kg
(TPC) y 20,5 mmol TE/Kg (DPPH), con quercetin-3-o-glucuronido (0,43 g/Kg) como
el principal compuesto fenólico entre los ocho identificados. Los resultados
destacan el potencial de los solventes verdes para obtener compuestos fenólicos
de alta capacidad antioxidante potencialmente bioactivos a partir de materias
primas de bajo costo.
Palabras claves:
extracción
verde, compuestos bioactivos, taninos hidrolizables, flavonoles, antioxidantes
naturales
Originales:
Recepción: 25/10/2023 - Aceptación: 26/12/2023
Introduction
One-third of
fruit and vegetable production is wasted or lost in the production chain,
producing avoidable and non-avoidable waste (3, 31). The former
includes the losses generated by wrong handling during postharvest, processing,
transport, storage, and distribution, and the non-avoidable waste is that part
of the vegetable or fruit that must be eliminated for processing and sale
(peels, seeds, core, and inedible parts). Therefore, the appropriate disposal
of this waste is essential to reduce the environmental and economic impact of
agro-industrial activity. The circular economy proposes different solutions to
prevent this waste plant tissue from ending up in city landfills (35). Although the
composition of fruit and vegetable by-products includes vitamins, minerals, and
carbohydrates (27), as well as
different bioactive compounds like phenolic compounds, these residual plant tissues
are mainly used as ingredients in animal feed or as a source of energy for
boilers. Waste fruit and vegetable tissues are sources of phenolic compounds
with significant health benefits for consumers and antioxidant properties (24,
33).
Phenolic compounds are synthesised in the plant´s secondary metabolism during
the normal development of plants. Their chemical structure has at least one
aromatic ring with one or more hydroxyl groups, showing different biological
activities according to their carbon bridges and hydroxyl substitution.
The production,
consumption and industrial processing of apples and strawberries continue to
increase worldwide, and so do the wasted tissues associated (12). Non-edible or
non-processable fruit parts may have better composition and bioactivity than
edible tissues (5, 34). The main
phenolic compounds of strawberries are ellagitannins, phenolic acids and
flavonoids like anthocyanins (32), and the
principal apple phenolic compounds are catechins and proanthocyanidins (17), depending on
the cultivar, the area, and the type of production of these crops. The
extraction method of these compounds significantly impacts the extraction
yields and their bioactive potential. Considering the structural diversity of
the phenolic compounds, no single extraction system could produce the total
recovery of these metabolites of interest from all vegetable tissues.
The solid-liquid
extraction is commonly used to obtain phenolic compounds, and the success of
this process depends on the plant matrix, kind of solvent, temperature, pH, and
extraction technology. Polar protic solvents, like ethanol and methanol, can
donate hydrogen bonds (33). Otherwise,
polar aprotic solvents (acetone and ethyl acetate) have dipoles but do not
possess hydrogen atoms bonded with a high electronegativity atom that can be
donated in a hydrogen bond. Finally, apolar solvents (hexane and petroleum
ether) have bonds between atoms with similar electronegativities (33). The
extraction technique also affects phenolic compound yield. The Soxhlet
extraction technique is widely used for obtaining secondary metabolites from
different plant tissues, being a reference method for comparing the performance
of other extraction techniques. The Soxhlet extraction has good extraction
yields without using large quantities of solvent, with a total extraction time
of 1-6h, including multiple extraction cycles. This technique commonly employs
flammable, hazardous, and toxic organic solvents. The low production cost of
ethanol (by fermentation from renewable sources), low energy requirements for
final disposal and moderate chronic toxicity make it a sustainable option to
replace traditional solvents, along with water, considered the greenest
solvent. Water application is generally limited to low-polarity metabolites;
nevertheless, it is possible to broaden the solubilisation spectrum of wáter
with suitable parameters (8). Therefore,
green extraction with safe and non-toxic solvents, like water, ethanol, and
binary ethanol-water mixtures, must be studied. Both solvents are considered
safe and acceptable for use in the food industry by regulatory agencies (FDA).
The ethanol-aqueous solutions for polyphenol extraction from plant tissues have
better yields due to their azeotropic behaviour (1). Additionally,
safe solvents in Soxhlet extraction could also yield higher amounts of phenolic
compounds from some agro-industrial waste (7, 8, 26).
Complementary
treatments with phenolic compounds that strengthen the immune system or their
use as antioxidant agents would promote obtaining these phenolic compounds of
interest at a low cost, facilitating the accessibility to a larger population
and valuing the wasted agro-industrial tissues using green technologies.
Therefore, this work aims to study the extraction of the phenolic compounds
from strawberry by-products and ‘Granny Smith’ apple peel, characterise the
obtained extracts using the Soxhlet method, evaluate the impact of two green
solvents (water and ethanol 80%), and different solid-liquid ratios on the
total phenolic content, phenolic profile, and in-vitro antioxidant activity of
the extracts. have bonds between atoms with similar electronegativities (33). The
extraction technique also affects phenolic compound yield. The Soxhlet
extraction technique is widely used for obtaining secondary metabolites from
different plant tissues, being a reference method for comparing the performance
of other extraction techniques. The Soxhlet extraction has good extraction
yields without using large quantities of solvent, with a total extraction time
of 1-6h, including multiple extraction cycles. This technique commonly employs
flammable, hazardous, and toxic organic solvents. The low production cost of
ethanol (by fermentation from renewable sources), low energy requirements for
final disposal and moderate chronic toxicity make it a sustainable option to
replace traditional solvents, along with water, considered the greenest
solvent. Water application is generally limited to low-polarity metabolites;
nevertheless, it is possible to broaden the solubilisation spectrum of water
with suitable parameters (8). Therefore,
green extraction with safe and non-toxic solvents, like water, ethanol, and
binary ethanol-water mixtures, must be studied. Both solvents are considered
safe and acceptable for use in the food industry by regulatory agencies (FDA).
The ethanol-aqueous solutions for polyphenol extraction from plant tissues have
better yields due to their azeotropic behaviour (1). Additionally,
safe solvents in Soxhlet extraction could also yield higher amounts of phenolic
compounds from some agro-industrial waste (7, 8, 26).
Complementary
treatments with phenolic compounds that strengthen the immune system or their
use as antioxidant agents would promote obtaining these phenolic compounds of
interest at a low cost, facilitating the accessibility to a larger population
and valuing the wasted agro-industrial tissues using green technologies.
Therefore, this work aims to study the extraction of the phenolic compounds
from strawberry by-products and ‘Granny Smith’ apple peel, characterise the
obtained extracts using the Soxhlet method, evaluate the impact of two green
solvents (water and ethanol 80%), and different solid-liquid ratios on the
total phenolic content, phenolic profile, and in-vitro antioxidant activity of
the extracts.
Materials
and methods
Plant
material and Experimental design
The by-products
of strawberry (RF) (Fragaria x ananassa Duch) cv ‘Festival’, consisting
of sepals and stems, with non-processable parts of the fruit (part of the fruit
closest to the sepal and peduncle), came from a single field (Coronda, Santa
Fe, Argentina) during postharvest preparation for industrial processing.
‘Granny Smith’ apple peel (GS, 1 mm thickness) was obtained from the minimal
processing of apples, according to Rodríguez-Arzuaga
and Piagentini (2018).
The RF and GS samples (89.2% and 80.4% moisture content, respectively) were
weighed, packed in 40 μm polyethylene bags, and stored at -20°C until
processing. Before extraction assays, both samples were ground to a particle
size <1 mm.
The effect of
the extraction solvent (S) and the solid-liquid ratio (R) in the phenolic compound
extraction of each vegetable tissue (RF and GS) was studied through a factorial
experimental design. The two experimental variables of each factorial design
were S and R, with two [S: water (W) and ethanol 80% (EtOH)] and four levels
[R: 1:10, 1:20, 1:30 and 1:40 w/v], respectively. The total phenolic content
(TPC), phenolic compound profile, and antioxidant capacity (DPPH) were
determined on each extract of RF and GS. The extraction times were four hours
for EtOH extractions and eight hours for W extractions (extraction times
determined in preliminary assays). The extracts were cooled (20°C), filtered,
and stored at -20°C for further analysis. Each phenolic compound extraction
assay was performed in triplicate.
Total
phenolic content (TPC)
Each extract TPC
was determined in triplicate by the Folin-Ciocalteu method (34). Gallic acid
(Sigma-Aldrich, San Luis, Missouri, USA) was used as the standard reagent to
perform the calibration curve, measuring the absorbance of the reaction at 760
nm in a spectrophotometer (Genesys 10s UV-Vis, Thermo Scientific™, Waltham,
Massachusetts, USA). The concentration of TPC was reported as g of gallic acid
equivalent (GAE) per kilogram of RF or GS (g GAE/Kg).
Phenolic
compound profile
The phenolic
compound profile of the extracts was performed with an LC-20AT HPLC with a
photodiode array detector (PAD), with the software Lab Solutions for data
processing and control (Shimadzu Co., Kyoto, Japan). The separation was
performed with a hybrid reverse phase column C18 Gemini 5μ 110Å of 250×4.6 mm,
attached to a guard column (Phenomenex Inc, CA, USA). The analysis was
performed according to Villamil-Galindo et al. (2021) for RF and Villamil-Galindo
and Piagentini (2022a)
for GS. The quantification of the identified compounds was performed using the
following external standards (Sigma-Aldrich Inc. St. Louis, Missouri, USA):
Ellagic acid (EA), Kaempferol-3-O-glucoside (K3G), Quercetin-3-O-glucoside
(Q3G), Chlorogenic acid (ACl), Procyanidin B2 (PACB2), (-) Epicatechin (EPQN),
(+) Catechin (CQN), Floretin (FLN), Gallic acid (GA), Coumaric acid (CUA), and
Ferulic acid (FRA). The results were expressed in g per Kg of tissue
by-product.
Antioxidant
capacity (DPPH)
The extract DPPH
was determined using the 2.2-diphenyl-1-picrylhydrazyl radical (DPPH*)
scavenging assay, performed by triplicate (34). A volume (200
μL) of the DPPH* methanolic solution (0.08 mM) reacts with 25 μL of extract or
reference reagent 2,5,7,8-tetramethylchroman-2-carboxylic
acid (Trolox, Sigma-Aldrich), and the absorbance was measured at 515 nm in a
microplate reader (Asus UVM 340, Cambridge, England) after 2 h. The results
were expressed as mmol Trolox equivalents/Kg of tissue by-product (mmol TE/Kg).
Statistical
analysis
All assays were
carried out in triplicates, and data were presented as the mean ± standard
deviation (SD). The effect of the solid-liquid ratio and the extraction solvent
on the total phenolic content, phenolic compound profile and antioxidant
capacity of RS and GS extracts were determined by the analysis of variance
(ANOVA). Tukey´s test (5% significance level) was used to determine the
significant differences among treatment means. Besides, the correlation between
the individually identified phenolic compounds and the antioxidant capacity
determined in each extract was determined using Pearson’s correlation test. The
statistical analysis was performed with STATGRAPHICS Centurion XV software
(StatPoint Technologies Inc., Warrenton, VA, USA).
Results
and discussion
Phenolic
compounds recovery from strawberry agro-industrial by-products (RF)
Both Soxhlet
extraction parameters [solid-liquid ratio (R) and type of solvent (S)] affected
(p<0.001) the yields of total phenolic content (TPC), the phenolic
compounds profile, and the antioxidant capacity (DPPH) of the strawberry
agro-industrial by-products extracts. The interaction term between R and S was
also significant (p<0.001), meaning that R affected in a different
way TPC, DPPH, and the phenolic compounds profile of the extracts depending on
the type of solvent used.
The increase of
R values improved the TPC yields of RF Soxhlet extraction with wáter (W), with
the highest yield (10.69 g GAE/Kg) obtained at R 1:40, up to 48% higher than those
obtained at lower R values (table 1).
Table
1. Total phenolic content (TPC) and
antioxidant capacity (DPPH) of the extracts from strawberry by-products (RF)
and ‘Granny Smith’ apple peel (GS).
Tabla 1. Contenido
de fenoles totales (TPC) y capacidad antioxidante (DPPH) de los extractos de
sub-productos de frutilla (RF) y cáscara de manzana ‘Granny Smith’ (GS).
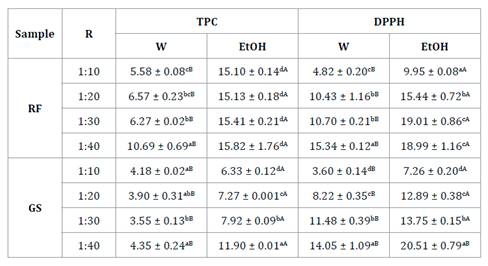
Mean ± standard deviation. R: Solid-liquid ratio. W:
water; EtOH: 80% ethanol. Different capital letters and lowercase letters
indicate significant differences (p<0.05) by Tukey’s test, between solvent
and among different solid-liquid ratios, respectively.
Promedio ± desviación estándard. R: relación
sólido-líquido. W: agua; EtOH: 80% etanol. Letras mayúscula y minúsculas
indican diferencias significativas (p<0,05) por el test de Tukey, entre
solvente y entre diferentes relaciones sólido-líquido, respectivamente.
The
concentration gradient between the solute and solvent was the driving force for
the diffusion process. Therefore, better extraction yields were obtained with
the highest R-value (1:40). Therefore, strawberry by-products have great
potential as a source of bioactive compounds obtained with water.
The RF Soxhlet
extraction with EtOH significantly increased the recovery of TPC as compared
with W by 47-170% (table 1). Contrary to W
extractions, R did not affect the TPC yields of the EtOH extracts (mean value
15.37 g GAE/Kg) (table 1). The phenolic compounds of RF could
present preferential solvation when using water-ethanol. The compounds would
show hydrophobic hydration with the few polar groups they could have in their
structure, and a large amount of (-OH) groups would allow more interaction with
water (6). Therefore,
this phenomenon would produce more effect than the increase in the extraction
ratio. However, the use of EtOH on the Soxhlet extraction system significantly
increased the TPC yields compared to RF water extracts, improving extraction
yields by up to 170% (table 1). Therefore,
the lower the extraction ratio, the smaller the solvent needed, and the lower
the production costs of the extracts.
Furthermore,
these results confirm that binary ethanol-water mixtures are suitable
bio-solvents for obtaining phenolic compounds due to their polar protic
properties. The yields obtained for RF with EtOH using Soxhlet extraction were
higher than those reported for pandan leaves (6.6 g GAE/Kg), mango by-products
(4.5-6.6 g GAE/Kg), asparagus (2.8-3.7 g GAE/Kg), cauliflower (1.1-1.8 g
GAE/Kg), and bergamot lemon (4-10 g GAE/Kg) (4, 14, 15, 27).
Regarding the
antioxidant capacity of RF water extracts, they were significantly affected by
R-value, comparable to TPC. The highest DPPH value was obtained for the 1:40
ratio (15.3 mmol TE/Kg), being up to 69% higher than that obtained at R 1:10.
Otherwise, like with the content of phenolic compounds, EtOH improved the
antioxidant capacity of the RF extracts obtained compared with W extracts. The
EtOH RF extracts with the highest DPPH values (18.9-19.0 mmol TE/Kg) were those
obtained with R 1:40 and 1:30 (p>0.05), respectively. The phenolic compounds
are excellent antioxidants of natural origin due to their reducing- capacity,
shown by the highly significant correlation between the TPC and the antioxidant
capacity of the RF extracts.
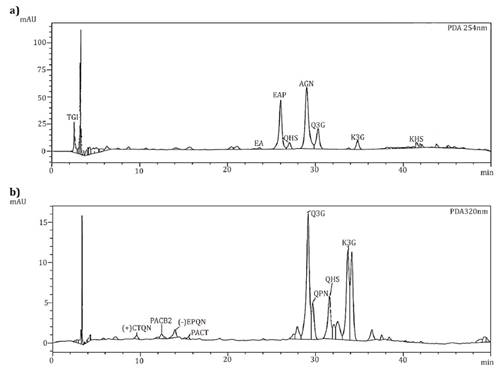
Eight major phenolic
compounds were identified for the strawberry agro-industrial waste tissue (RF),
belonging to three main phenolic compound classes: hydrolysable tannins,
ellagic acid derivatives, and flavonols (figure 1a and figure
2).
TGI:
Tetragalloyglucose isomer, EAP: Ellagic acid pentoxide, AGN:
Dimer of galloyl-bis-HHDPglucose (agrimoniin isomer), EA: Ellagic acid, Q3G:
Quercetin- 3-O-glucuronide, QHS: Quercetin Hexoxide, K3G:
Kaempferol-3- O-glucuronide. (+) CTQN: Catechin, PACB2:
Procyanidin B2, (-) EPQN: Epicatechin, PACT: Procyanidin
tetramer, QPN: Quercetin pentoxide.
TGI:
Tetragalloyglucosa isomero, EAP: pentoxidode ácido Ellagico, AGN:
Dimero de galloylbis- HHDP-glucosa (agrimoniin isomero), EA: ácido
Ellagico, Q3G: Quercetin-3- O-glucuronido, QHS: Quercetin
Hexoxido, K3G: Kaempferol-3-Oglucuronido. (+) CTQN:
Catequina, PACB2: Procianidina B2, (-) EPQN: Epicatequina, PACT:
Procianidin tetramero, QPN: Quercetina pentoxido.
Figure 1. Typical
HPLC-UV chromatogram obtained of (a) strawberry by-products at 254 nm and (b)
‘Granny Smith’ apple peel at 320 nm.
Figura 1. Cromatograma
típico HPLC-UV obtenido para (a) sub-productos de frutillas a 254 nm y (b)
cáscara de manzanas ‘Granny Smith’ a 320 nm.
TGI:
Tetragalloyglucose isomer, EAP: Ellagic acid pentoxide, AGN:
Dimer of galloyl-bis-HHDPglucose (agrimoniin isomer), EA: Ellagic acid, Q3G:
Quercetin- 3-O-glucuronide, QHS: Quercetin Hexoxide, K3G:
Kaempferol- 3-O-glucuronide, TPCHPLC: Total phenolic compounds
analyzed by HPLC. Different lowercase letters indicate significant differences
(p<0.05) by Tukey´s test, between different solidliquid ratios.
TGI:
Tetragalloyglucosa isomero, EAP: pentoxido de ácido Ellagico, AGN:
Dimero de galloylbis- HHDP-glucosa (agrimoniin isomero), EA: ácido
Ellagico, Q3G: Quercetin-3- O-glucuronido, QHS: Quercetin
Hexoxido, K3G: Kaempferol-3-Oglucuronido, TPCHPLC:
Compuestos fenólicos totales analizados por HPLC. Diferentes letras minúsculas
indican diferencias significativas (p<0,05) por el test de Tukey, entre
diferentes relaciones sólido-líquido.
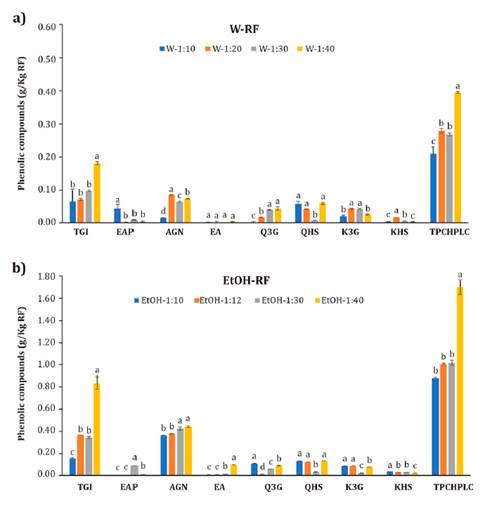
Figure 2. Phenolic
compounds from strawberry by‑products (RF) extracted with a) wáter (W) and b)
80% ethanol (EtOH).
Figura 2. Compuestos
fenólicos de los sub-productos de frutilla extraídos con a) agua (W) and b) 80%
etanol (EtOH).
Tetragalloyl-glucose
isomer (TGI) and galloyl-bis-HHDP-glucose dimer (agrimoniin) (AGN) were
identified among the hydrolysable tannins; ellagic acid pentoxide (EAP) and
free ellagic acid (EA) among the ellagic acid derivatives; and finally, the
flavonols were represented by quercetin-3-O-glucuronide (Q3G), quercetin
hexoxide (QHS), kaempferol-3-O-glucuronide (K3G), and kaempferol hexoxide
(KHS).
For the water
extracts at R 1:10, hydrolysable tannins represented 38.5% of the total
phenolic compounds, similar to flavonols with 39.9%, followed by ellagic acid
derivatives with 21.6% (figure 2a). However, as
the extraction ratio increased, hydrolysable tannins represented 64% of the
total phenolic compounds in the extracts obtained with R 1:40, showing that the
increase in the concentration gradient in the extraction solvent (W) allowed
greater recovery of hydrolysable tannins (33).
The highest
concentration of tetragalloylglucose isomer (TGI) in W extracts was obtained at
R 1:40 (0.18 g/Kg); for the other R-values, the TGI concentration was similar
(0.07-0.1 g/Kg) (figure 2a). EtOH extraction significantly
improved the TGI yields for all extraction ratios, with the maximum
concentration at R 1:40 (0.83 g/Kg). The Ellagic acid pentoxide (EAP) had the
highest concentration, 0.088 g/Kg (p<0.05), in the EtOH extracts (figure
2).
EAP concentrations were higher than those reported for the stem of Sanguisorba
Officinalis L (Rosaceae family) (0.038 g/Kg) (20). Agrimoniin
(AGN) is a compound derived from hexahydroxydiphenic acid (HHDP), considered a
taxonomic marker of the Rosaceae, with great importance in the nutraceutical
industry due to its bioactive properties (30). The AGN yield
obtained with water was about 0.064-0.084 g/kg for the higher R values. The AGN
yields were significantly improved for all R-values (p<0.05) when EtOH was
used, with a maximum concentration of 0.44 g/Kg at R 1:40 (figure 2). This AGN
concentration was higher than the reported for whole strawberry fruit extracts
(0.12 g/Kg) obtained with 70% methanol (25), showing this
residual tissue as a valuable source of AGN. The antioxidant properties of
hydrolysable tannins have been reported in-vitro and in-vivo (16). Simirgiotis
et al. (2010)
reported that cyanidin glucosides and ellagic acid were the compounds with the
highest participation in the antioxidant activity in the edible part of
strawberries. For RF, the hydrolysable tannins TGI and AGN had a significant
correlation (p<0.01) with the DPPH* antioxidant capacity. These compounds
have polyhydric alcohol in the centre, and their hydroxyl groups could be
partially esterified with ellagic acid or HHDP, having the capacity to yield
electrons and thus neutralise the free radicals present (14). The ellagic
acid (EA) concentrations were lower in W extracts for any extraction ratio
(p>0.05). Like AGN, EtOH improved the extraction of EA, with a maximum
concentration of 0.10 g/Kg R 1:40 (figure 2). The
consumption of ellagic acid derivatives was associated with numerous health
benefits since, in-vivo conditions, different types of urolithins were
metabolised by the microbiota, which had powerful antiproliferative and cancer
cell apoptosis-inducing activities (21).
The flavonol
Quercetin-3-o-glucuronide (Q3G) concentrations in RF water extracts were
0.002-0.04 g/Kg, obtaining the higher at R 1:40 and 1:30 (p>0.05).
Nevertheless, using 80% ethanol significantly increased Q3G yields up to 0.11
g/Kg (figure
2),
similar to chokeberry extracts (11). QHS
concentrations obtained with EtOH (0.12-0.13 g/Kg) were higher than those
obtained with W (0.04-0.05 g/Kg). Kaempferol is one of the most common
flavonols in different botanical species. In the cell vacuoles, Kaempferol
tends to glycosidate with some carbohydrates to have more stability in the pH
of the medium (28). The highest
kaempferol-3-o-glucuronide (K3G) concentration obtained with water was 0.043
g/Kg. EtOH improved the K3G extraction yields by about 50% (figure 2). K3G values in
RF were close to those reported for green tea (60% methanol), a popular
antioxidant infusion (19). Finally, the
Kamepferol Hexoxide (KHS) yield increased by up to 91% in EtOH extractions
compared to water extracts (figure 2).
Therefore, the
agro-industrial strawberry by-product showed a large variability of phenolic
compounds of interest. The highest recovery of total phenolic compounds
(TPCHPLC) with W was achieved with R 1:40 (0.40 g/Kg). As expected,
the EtOH increased TPCHPLC up to 425%, obtaining the highest concentration also
at R 1:40 (figure
2).
Similar to the results of TPC and DPPH, the extractions with the ethanol-water
binary mixture (80:20) had the highest recovery of phenolic compounds with high
antioxidant capacity. The máximum TPCHPLC content (1.70 g/Kg) obtained for RF
EtOH extracts was comparable to that reported for strawberry plant leaves
(1.95-2.07 g/Kg) obtained with methanol-formic acid (99:1) (30). The phenolic
compounds of RF have an excellent antioxidant, anti-inflammatory and
anticarcinogenic potential for colorectal cancer (34, 36). Therefore,
the high concentration of hydrolysable tannins and ellagic acid derivatives in
RF enables the promotion of this kind of agro-industrial by-products as a
low-cost source of healthy compounds.
Phenolic
compounds recovery from ‘Granny Smith’ apple peel (GS).
The solid-liquid
ratio (R) and the type of solvent (S) affected (p<0.001) the content of
phenolic compounds and the antioxidant capacity of GS extracts. The interaction
term between R and S was also significant (p<0.001) for TPC, DPPH, and
phenolic compounds profile.
The use of EtOH
improved the TPC recovery from GS, like RF (table 1). The TPC of GS
EtOH extracts increased as R increased, obtaining the highest yield (11.9 g
GAE/Kg) for R 1:40. Castro-López et al. (2017) reported that
R-values higher than 1:20 increased the recovery of phenolic compounds. Binary
alcohol-water mixtures offer an eco-friendly solvent system for obtaining
phenolic compounds from different wasted plant matrices than those using pure
ethanol or other organic solvents. The water and ethanol mixture act
synergistically and could provide a suitable polarity range for extracting
phenolic compounds (medium-high polarity). The former is fundamental as a
swelling agent of the plant matrix, allowing the lower viscosity ethanol to
diffuse through the material and break the non-covalent interactions between
the solute and the matrix, facilitating the preferential solvation sphere
transferring the analyte to the dissolution medium (33).
Phenolic
compounds are the plant-secondary metabolites with the highest reported
antioxidant activity. Each compound antioxidant capacity differs due to its
oxidation-reduction reactions, phenyl ring structure resonance, and hydroxyl
group substitution pattern (32). The
antioxidant capacity of GS extracts at R 1:40 is 47% higher in EtOH extract
than in W extract. The DPPH values obtained were comparable to those reported
for other plant materials by Soxhlet extraction (sugar beet molasses, rapeseed,
and flowers of Jatropha integerrima) (10, 13). The use of
EtOH enhanced the antioxidant capacity of GS extracts as R increased (table
1),
comparable to TPC, and therefore, showing a strong correlation between TPC and
DPPH (p<0.01).
The two main
classes of phenolic compounds identified and quantified in ‘Granny Smith’ apple
peel (GS) were the flavan-3-ols with (+) catechin [(+) CTQN], Procyanidin B2
(PACB2), (-) epicatechin [(-) EPQN], and Procyanidin tetramer (PACT); and the
flavonols with the Quercetin-3-o-glucuronide (Q3G), Quercetin pentoxide (QPN),
Quercetin Hexoxide (QHS), and Kaempferol-3-o-glucuronide (K3G) (figure
1b
and figure
3).
(+)CTQN:
Catechin, PACB2: Procyanidin B2, (-)EPQN:
Epicatechin, PACT: Procyanidin tetramer, Q3G: Quercetin- 3-O-glucuronide,
QPN: Quercetin pentoxide, QHS: Quercetin Hexoxide, K3G:
Kaempferol- 3-O-glucuronide, TPCHPLC: Total phenolic compounds
analyzed by HPLC. Different lowercase letters indicate significant differences
(p<0.05) by Tukey´s test, between different solid-liquid ratios.
(+)CTQN:
Catequina, PACB2: Procianidina B2, (-)EPQN:
Epicatequina, PACT: Procianidina tetramero, Q3G: Quercetina-3- O-glucuronido,
QPN: Quercetina pentoxido, QHS: Quercetina Hexoxido, K3G:
Kaempferol-3-Oglucuronido, TPCHPLC: Compuestos fenólicos totales
analizados por HPLC. Diferentes letras minúsculas indican diferencias
significativas (p<0,05) por el test de Tukey, entre diferentes relaciones
sólidolíquido.
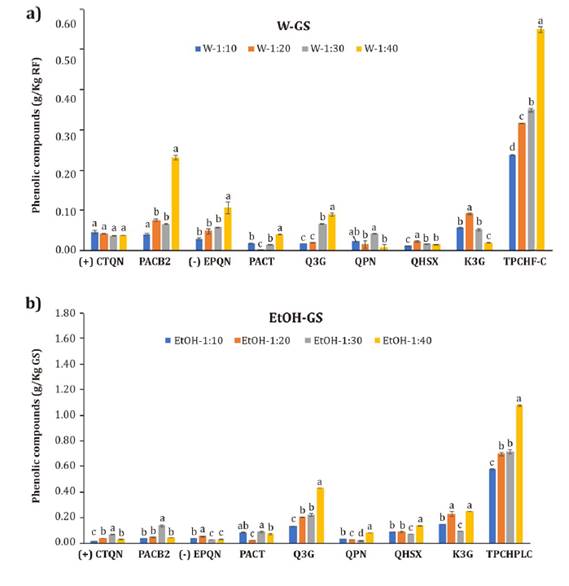
Figure 3. Phenolic
compounds from ‘Granny Smith’ apple peel (GS) extracted with a) water (W) and
b) 80% ethanol (EtOH).
Figura 3. Compuestos
fenólicos de cáscara de manzana compounds from ‘Granny Smith’ (GS) extraidos
con a) agua (W) and b) 80% etanol (EtOH).
For the GS W
extracts, the flavan-3-ols and flavanols represented each 50 % of the total
phenolic compounds (R 1:10, 1:20 and 1:30), increasing the flavan-3-ols
proportion with R 1:40 up to 76 % of the total of the quantified compounds,
showing the affinity of this class of phenolic compounds for a polar solvent
like water (figure 3a). Nevertheless, flavonols accounted for
more than 50% of the total phenolic compounds in all the EtOH extractions, with
a maximum of 84% in the extracts with R 1:40 (figure 3b).
R did not affect
(p>0.05) the (+)CTQN extraction yield with water.
EtOH extractions increased (+)CTQN yields up to 0.066
g/kg, 77% higher than W extracts (figure 3). The (+)CTQN yields obtained were lower than those reported by Almeida
et al. (2017)
for ‘Granny Smith’ apple peel extracted with 100% acetone (0.17 g/Kg). PACB2
(epicatechin-epicatechin dimer) is the most common proanthocyanidin determined
in high concentrations in fruits like peaches, apples, and plums. The PACB2
content in W extracts increased with R; it was at least 71% higher for R 1:40
than for the other extraction ratios. However, ethanol did not improve the
PACB2 extraction yields. Procyanidin B2 in a liquid medium from 90°C onwards
starts a degradation process by oxidation and epimerisation, lowering the
procyanidin B2 recovery. The highest concentration of EPQN, reported as the
main phenolic compound in apples (23), was achieved
with W and R 1:40 (0.1 g/Kg), being higher than that reported for apple pomace
extracts (0.02 g/Kg) (20). The highest
PACT concentration in W extracts was 0.04 g/Kg, obtained at R 1:40. The EtOH
improved PACT yields (p<0.05) for all R values (figure 3). Procyanidins
are oligomers composed of catechin and epicatechin; their structure and high
molecular weight give them different bioactive and functional properties for
the food industry.
Q3G extraction
yields with W were affected by R, obtaining the highest one at R 1:40, 82%
higher than the yields obtained at lower R values. Nevertheless, the EtOH
improved Q3G extraction yields, as expected for a medium polarity compound. The
highest Q3G yield with EtOH (0.43 g/Kg) was obtained at R 1:40, 50% higher than
those obtained at lower R values (figure 3). Moreover, Q3G
was the GS individual compound with the highest correlation with the
antioxidant capacity, mainly for its structure and hydroxyl groups that allowed
the donation of electrons, neutralising the free radicals present in the medium
(32). Contrarily, RF flavonols did not show
a highly significant correlation with the antioxidant capacity determined by
DPPH. Consequently, the bioactive potential did not depend only on the
bioactive compound concentration but also on the interaction with the food
matrix. The values obtained with EtOH were similar and even higher than those
reported for ‘Granny Smith’ apple peel acetone extracts (0.18-0.4 g/Kg) (2).
The highest
concentration of QPN, the other quercetin glucoside derivative, was obtained
with EtOH at R 1:40 (0.08 g/Kg). The yields of QHS in EtOH extracts were higher
than in W, obtaining the highest concentration at R 1:40 (0.14 g/Kg). According
to previous reports, quercetin and its glycosides have a low bioavailability
(16-25%) due to their low water solubility and crystalline structure at body
temperatures (16). Like the
other flavonols, EtOH enhanced the recovery of K3G, with the highest
concentration (0.25 g/Kg) at R 1:40 (figure 3).
Considering the
sum of the compounds identified and quantified, the TPCHPLC obtained for W
extracts increased with R, determining the highest concentration in W at R 1:40
(0.55 g/Kg) (figure 3a). Higher TPCHPLC values were obtained
with EtOH and higher R values. TPCHPLC extracted with EtOH 1:40 (1.07 g/Kg) was
higher than that reported for the apple pulp (17), showing the
bioactive potential of apple peel. The GS TPCHPLC highly correlates with
antioxidant activity (R2 0.87), mainly due to flavonols. These results
encourage the integral use of the apple peel as a source of valuable compounds,
focusing on green solvents use with low environmental impact and cost (35).
Conclusions
There is a
growing demand for nutraceutical products of vegetable origin, as their
frequent consumption has been associated with a decreasing risk of having
chronic non-transmissible diseases. The market for nutraceutical compounds is
booming, and the extraction of bioactive compounds using clean solvents from
agro-industrial waste tissues, like the strawberry by-products and apple peel,
presents an opportunity to reduce costs and the environmental impact. The
conventional Soxhlet extraction technique has good yields, low complexity, and
high efficiency, allowing optimal use of natural resources, especially those
that are rejected for industrial processing, like the waste tissue produced
during the postharvest trimming of the strawberry (about 7-20% of the fruit
intended for industrial processing) and Granny Smith apple peel (about 12% of
the fruit intended for minimal processing).
This study
demonstrates that waste vegetable tissues can be transformed into valuable
phenolic compounds with antioxidant properties using eco-friendly solvents such
as water and ethanol. The extracts with the highest content of phenolic
compounds and antioxidant capacity were obtained for Soxhlet extraction with
80% ethanol and 1:40 extraction ratio for both the strawberry by-products (15.8
g GAE/Kg and 19 mmol TE/Kg) and the ‘Granny Smith’ apple peel (11.9 g GAE/Kg
and 20.5 mmol TE/ Kg). Additionally, eight main phenolic compounds were
identified and quantified in both waste tissues. The hydrolysable tannins, like
Tetragalloyglucose isomer (TGI: 0.83 g/Kg) and Dimer of
galloyl-bis-HHDP-glucose (agrimoniin isomer, AGN: 0.44 g/Kg), were the main phenolic
compounds extracted from RF, while flavonols accounted for 83.7% of the total
extracted phenolic compounds from GS, obtaining for Quercetin-3-O-glucuronide
the highest yield (Q3G: 0.43 g/Kg).
These results
demonstrated the importance of by-products as low-cost sources of bioactive
compounds with high nutraceutical potential through a circular process approach
in the fruit and vegetable industry. Currently, these bio-wastes are disposed
of in landfills without any use. The information obtained in this study
provides a pathway towards the integral use of strawberry and apple
by-products. The challenge is to continue studying the development of a
procedure for obtaining bioactive compounds from strawberry by-products and
‘Granny Smith’ apple peel with higher yields, shorter extraction times and
lower energy consumption, using more sustainable and efficient technologies
stimulating an integral use of these by-products.
Acknowledgments
The authors
acknowledge the Universidad Nacional del Litoral and the Agencia Santafesina de
Ciencia, Tecnología e Innovación (ASaCTei) (Santa Fe-Argentina) for financial
support through Projects CAI+D 2020 and PEICID-2022-177; the support of CONICET
(Argentina) from a doctoral grant; and María del Huerto Sordo for providing strawberry
by-products.
1. Alara, O.;
Abdurahman, N.; Ukaegbu, C. 2018. Soxhlet extraction of phenolic compounds from
Vernonia cinerea leaves and its antioxidant activity. Journal of Applied
Research on Medicinal and Aromatic Plants. 11: 12-17. https://doi.org/10.1016/j.jarmap.2018.07.003
2. Almeida, D.
P. F.; Gião, M. S., Pintado, M.; Gomes, M. H. 2017. Bioactive phytochemicals in
Apple cultivars from the Portuguese protected geographical indication “Maçã de
Alcobaça”: Basis for market segmentation. International Journal of Food
Properties. 20(10): 2206-2214. https://doi.org/10.1080/10942912.2016.1233431
3. Bigaran
Aliotte, J. T.; Ramos de Oliveira, A. L. 2022. Multicriteria decision analysis
for fruits and vegetables routes based on the food miles concept. Revista de la
Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 54(1): 97-108. DOI: https://doi.org/10.48162/rev.39.069
4.
Blancas-Benitez, F. J.; Mercado-Mercado, G.; Quirós-Sauceda, A. E.;
Montalvo-González, E.; González Aguilar, G. A.; Sáyago-Ayerdi, S. G. 2015.
Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics
release of polyphenols in Mexican “Ataulfo” mango (Mangifera indica L.)
by-products. Food and Function. 6(3): 859-868.
https://doi.org/10.1039/c4fo00982g
5. Boiteux, J.;
Fernández, M. de los Á.; Espino, M.; Silva, M. F.; Pizzuolo, P. H.; Lucero, G.
S. 2023. In vitro and in vivo efficacy of Larrea divaricata extract
for the management of Phytophthora palmivora in olive trees. Revista de
la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 55(2): 97-107. DOI: https://doi.org/10.48162/rev.39.112
6. Bosch, E.;
Rosés, M. 1992. Relationship between ET polarity and composition in binary
solvent mixtures. Journal of the Chemical Society, Faraday Transactions.
88(24): 3541-3546. https://doi.org/10.1039/FT9928803541
7. Bucić-Kojić,
A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. 2007. Study of solid-liquid
extraction kinetics of total polyphenols from grape seeds. Journal of Food
Engineering. 81(1): 236-242. https://doi.org/10.1016/j.jfoodeng.2006.10.027
8. Carciochi, R.
A.; Manrique, G. D.; Dimitrov, K. 2015. Optimization of antioxidant phenolic
compounds extraction from quinoa (Chenopodium quinoa) seeds. Journal of
Food Science and Technology. 52(7): 4396-4404.
https://doi.org/10.1007/s13197-014-1514-4
9. Castro-López,
C.; Ventura-Sobrevilla, J. M.; González-Hernández, M. D.; Rojas, R.; Ascacio-Valdés,
J. A.; Aguilar, C. N.; Martínez-Ávila, G. C. G. 2017. Impact of extraction
techniques on antioxidant capacities and phytochemical composition of
polyphenol-rich extracts. Food Chemistry. 237: 1139-1148.
https://doi.org/10.1016/j.foodchem.2017.06.032
10. Chen, M.;
Zhao, Y.; Yu, S. 2015. Optimisation of ultrasonic-assisted extraction of
phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses.
Food Chemistry. 172: 543-550. https://doi.org/10.1016/j.foodchem.2014.09.110
11. Ćujić, N.;
Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. 2016.
Optimization of polyphenols extraction from dried chokeberry using maceration
as traditional technique. Food Chemistry. 194: 135-142.
https://doi.org/10.1016/j.foodchem.2015.08.008
12. del Brio,
D.; Tassile, V.; Bramardi, S. J.; Fernández, D. E.; Reeb, P. D. 2023. Apple (Malus
domestica) and pear (Pyrus communis) yield prediction after tree
image analysis. Revista de la Facultad de Ciencias Agrarias. Universidad
Nacional de Cuyo. Mendoza. Argentina. 55(2): 1-11. DOI:
https://doi.org/10.48162/rev.39.104
13. Feng, X.;
Chenyi, L.; Jia, X.; Guo, Y.; Lei, N.; Hackman, R.; Chen, L.; Zhou, G. H. 2016.
Influence of sodium nitrite on protein oxidation and nitrosation of sausages
subjected to processing and storage. Meat Science. 116.
https://doi.org/10.1016/j.meatsci.2016.01.017
14. Ghasemzadeh,
A.; Jaafar, H. Z. E. 2014. Optimization of reflux conditions for total
flavonoid and total phenolic extraction and enhanced antioxidant capacity in
Pandan (Pandanus amaryllifolius Roxb.) using response surface
methodology. The Scientific World Journal. 523120.
https://doi.org/10.1155/2014/523120
15. Goni, I.;
Hervert-Hernandez, D. 2011. By-Products from Plant Foods are Sources of Dietary
Fibre and Antioxidants. Phytochemicals - Bioactivities and Impact on Health.
https://doi.org/10.5772/27923
16. Grochowski,
D. M.; Skalicka-Woźniak, K.; Orhan, I. E.; Xiao, J.; Locatelli, M.; Piwowarski,
J. P.; Granica, S.; Tomczyk, M. 2017. A comprehensive review of agrimoniin.
Annals of the New York Academy of Sciences. 1401(1): 166-180.
https://doi.org/10.1111/nyas.13421
17. Kalinowska,
M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W.
2014. Apples: Content of phenolic compounds vs. variety,
part of apple and cultivation model, extraction of phenolic compounds,
biological properties. Plant Physiology and Biochemistry. 84: 169e188-188.
https://doi.org/10.1016/j.plaphy.2014.09.006
18. Kasikci, M.
B.; Bagdatlioglu, N. 2016. Bioavailability of quercetin. Current Research in
Nutrition and Food Science. 4: 146-151. https://doi.org/10.12944/CRNFSJ.4.Special-Issue-October.20
19. Kingori, S.
M.; Ochanda, S. O.; Koech, R. K. 2021. Variation in Levels of Flavonols
Myricetin, Quercetin and Kaempferol - In Kenyan Tea (Camellia sinensis L.)
with Processed Tea Types and Geographic Location. Open Journal of Applied
Sciences. 11: 736-749. https://doi.org/10.4236/ojapps.2021.116054
20. Lachowicz,
S.; Oszmiański, J.; Rapak, A.; Ochmian, I. 2020. Profile and content of
phenolic compounds in leaves, flowers, roots, and stalks of sanguisorba
officinalis l. Determined with the LC-DAD-ESI- QTOF-MS/MS analysis and their in
vitro antioxidant, antidiabetic, antiproliferative potency.
Pharmaceuticals. 13(8): 1-22. https://doi.org/10.3390/ph13080191
21. Landete, J.
M. 2011. Ellagitannins, ellagic acid and their derived metabolites: A review
about source, metabolism, functions and health. Food Research International.
44(5): 1150-1160. https://doi.org/10.1016/j.foodres.2011.04.027
22. Li, W.;
Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M. A. 2020. Analysis of
polyphenols in Apple pomace: A comparative study of different extraction and
hydrolysis procedures. Industrial Crops and Products. 147.
https://doi.org/10.1016/j.indcrop.2020.112250
23. Lončarić,
A.; Matanović, K.; Ferrer, P.; Kovač, T.; Šarkanj, B.; Babojelić, M. S.; Lores,
M. 2020. Peel of traditional apple varieties as a great source of bioactive
compounds: Extraction by micromatrix solid-phase dispersion. Foods. 9(1): 4-6.
https://doi.org/10.3390/foods9010080
24.
Mercado-Mercado, G.; Montalvo-González, E.; González-Aguilar, G. A.;
Alvarez-Parrilla, E.; Sáyago-Ayerdi, S. G. 2018. Ultrasound-assisted extraction
of carotenoids from mango (Mangifera indica L. ‘Ataulfo’) by-products on
in vitro bioaccessibility. Food Bioscience. 21: 125-131.
https://doi.org/10.1016/j.fbio.2017.12.012
25. Nowicka, A.;
Kucharska, A. Z.; Sokół-Łętowska, A.; Fecka, I. 2019. Comparison of polyphenol
content and antioxidant capacity of strawberry fruit from 90 cultivars of
Fragaria × ananassa Duch. Food Chemistry. 270: 32-46.
https://doi.org/10.1016/j.foodchem.2018.07.015
26. Oszmiański,
J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. 2011. Identification and
characterization of low molecular weight polyphenols in berry leaf extract by
HPLC-DAD and LC-ESI/MS. Journal of Agricultural and Food Chemistry. 59:
12830-12835. https://doi.org/10.1021/jf203052j
27. Putnik, P.;
Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F. J.; Cravotto, G.; Binello,
A.; Lorenzo, J. M.; Shpigelman, A. 2017. Innovative “green” and novel
strategies for the extraction of bioactive added value compounds from citrus
wastes - A review. Molecules. 22(5). https://doi.org/10.3390/molecules22050680
28. Rana, R.;
Gulliya, B. 2019. Chemistry and Pharmacology of Flavonoids- A Review. Indian
Journal of Pharmaceutical Education and Research. 53: 8-20.
https://doi.org/10.5530/ijper.53.1.3
29.
Rodríguez-Arzuaga, M.; Piagentini, A. M. 2018. New antioxidant treatment with
yerba mate (Ilex paraguariensis) infusion for fresh-cut apples:
Modeling, optimization, and acceptability. Food Science and Technology
International. 24(3): 223-231. https://doi.org/10.1177/1082013217744424.
30. Simirgiotis,
M. J.; Schmeda-Hirschmann, G. 2010. Determination of phenolic composition and
antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria
chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free
radical quenching techniques. Journal of Food Composition and Analysis. 23(6):
545-553. https://doi.org/10.1016/j.jfca.2009.08.020
31. UNEP. 2021.
Food Waste Index Report 2021. United Nations Environment Programme, Nairobi.
https://www.unep.org/resources/report/unep-food-waste-indexreport-2021
(accessed 2 July 2022).
32. Van de
Velde, F.; Grace, M. H.; Esposito, D.; Pirovani, M. É.; Lila, M. A. 2016.
Quantitative comparison of phytochemical profile, antioxidant, and
anti-inflammatory properties of blackberry fruits adapted to Argentina. Journal
of Food Composition and Analysis. 47: 82-91.
https://doi.org/10.1016/J.JFCA.2016.01.008
33.
Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A. M. 2020. Extracts from
strawberry by-products rich in phenolic compounds reduce the activity of apple
polyphenol oxidase. LWT, 133(May); 110097.
https://doi.org/10.1016/j.lwt.2020.110097
34.
Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A. M. 2021. Strawberry
agro-industrial by-products as a source of bioactive compounds: effect of
cultivar on the phenolic profile and the antioxidant capacity. Bioresources and
Bioprocessing. 8(1): 61. https://doi.org/10.1186/s40643-021-00416-z
35.
Villamil-Galindo, E.; Piagentini, A. M. 2022a. Sequential ultrasound-assisted
extraction of pectin and phenolic compounds for the valorisation of ‘Granny
Smith’ apple peel. Food Bioscience. 49(August); 101958.
https://doi.org/10.1016/j.fbio.2022.101958
36.
Villamil-Galindo, E.; Antunes-Ricardo, M.; Piagentini, A. M.; Jacobo-Velázquez,
D. A. 2022b. Adding value to strawberry agro-industrial by-products through
ultraviolet A-induced biofortification of antioxidant and anti-inflammatory
phenolic compounds. Front. Nutr. 9:1080147. doi: 10.3389/fnut.2022.1080147.