Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Compensatory
Growth in Pinus ponderosa (Dougl Ex Laws) Plantations Under Early
Silvicultural Treatments
Crecimiento
compensatorio en plantaciones de Pinus ponderosa (Dougl Ex Laws) bajo
tratamientos silviculturales tempranos
Andrea Alejandra
Medina2,
Marcos Ancalao1,
Matías Horacio
Saihueque1
1Campo Anexo San Martín - IFAB (INTA - CONICET)- Instituto de
Investigaciones Forestales y Agropecuarias de Bariloche. Ruta Nacional N° 40 Km
1911. C. P. 8430. Paraje Las Golondrinas. Lago Puelo. Chubut. Argentina.
2Universidad Nacional del Comahue. Centro Regional Universitario
San Martín de los Andes (CRUSMA). Pasaje de la Paz 235. San Martín de los
Andes. C. P. 8370. Neuquén. Argentina.
*letourneau.federico@inta.gob.ar
Abstract
Early pruning and
thinning in Pinus ponderosa, plantations in Andean Patagonia triggered
compensatory growth, characterized by greater trunk growth and structural
adjustments. We used a factorial design and mixed-effects models to evaluate
stem growth, crown light dynamics, tracheid length (TL), foliar biomass (FB),
wood density (WD), and Huber values (Hv) five years after treatment. Trees
under combined pruning and thinning (PT) showed the greatest basal area
increment, indicating resource reallocation to supportive structures despite
early foliage loss. Pruned trees maintained higher Hv and achieved partial
recovery of FB. Tracheid elongation was greatest in treated trees, suggesting
accelerated xylem maturation, while WD remained unchanged. These results
demonstrate the structural plasticity of P. ponderosa, which maintains
hydraulic function and growth after canopy disturbance. Our findings provide
useful guidance for silvicultural planning in temperate plantations.
Keywords: compensatory
growth, hydraulic architecture, adaptive response
Resumen
Los tratamientos
tempranos de poda y raleo en plantaciones de Pinus ponderosa en la
Patagonia Andina provocaron respuestas de crecimiento compensatorio,
evidenciadas por un mayor desarrollo del fuste y ajustes estructurales.
Mediante un diseño factorial y modelos de efectos mixtos, se evaluaron el
crecimiento del tallo, la dinámica de luz en la copa, la longitud de traqueidas
(TL), la biomasa foliar, la densidad de la madera y la razón de Huber (Hv)
cinco años después del tratamiento. Los árboles sometidos al tratamiento
combinado de poda y raleo (PT) mostraron el mayor incremento en el área basal,
indicando una reasignación de recursos hacia estructuras de sostén a pesar de
la pérdida foliar inicial. Los árboles podados mantuvieron valores elevados de
Hv y lograron una recuperación intermedia de biomasa foliar, mientras que la
elongación de traqueidas fue mayor en los árboles tratados, lo que sugiere una
maduración acelerada del xilema. La densidad de la madera no se vio afectada.
Estos resultados demuestran la plasticidad estructural de P. ponderosa,
evidenciando su capacidad para mantener la funcionalidad hidráulica y sostener
el crecimiento ante modificaciones en la copa. Los hallazgos aportan
herramientas útiles para la planificación silvícola en plantaciones templadas.
Palabras clave: crecimiento
compensatorio, arquitectura hidráulica, respuesta adaptativa
Originales: Recepción: 20/11/2023 - Aceptación: 24/10/2025
Introduction
Pinus ponderosa is the most
widespread conifer species in forest plantations in Andean Patagonia, Argentina
(24). Its cultivation
in ecotone zones has government support for its establishment and silvicultural
management. This species shows intermediate growth and numerous basal branches
requiring pruning to reduce fire risk or improve wood quality. Forest managers
must apply these cultural practices at an early, pre-commercial thinning stage
for these cultural practices to be effective. However, researchers have not
fully clarified how pruning and thinning affect early tree growth and
development.
Previous studies
have explored the influence of pruning and planting density on Pinus
ponderosa’s growth and physiological performance. For Gyenge et
al. (2009, 2010) demonstrated that pruning temporarily reduces diameter growth,
while planting density significantly affects resource availability and
individual tree growth. Additionally, Gyenge et al. (2012) analyzed responses
to water stress under different competition levels, showing short- and
long-term physiological adjustments. Similarly, Martínez-Meier et al. (2015) highlighted that
intraspecific competition alters the wood structure in high-density stands,
increasing earlywood density and reducing the hydraulic efficiency of trees,
affecting their ability to respond to water stress conditions.
Cambial maturation
is a key process in woody plants. It produces secondary xylem composed of
tracheids and other cellular elements. Tracheid size is a key indicator of this
maturation, influencing both hydraulic and mechanical function (13). In P.
ponderosa from this region, tracheid length (TL) increases during the
transition from juvenile to mature wood (17,
34).
TL in conifers also
correlates with tracheid diameter (30). Together, these
traits determine water transport efficiency through the xylem. Therefore, TL
provides valuable information on cambial maturation and its impact on wood
function and quality.
Pine productivity
depends strongly on canopy structure, including crown shape, leaf area index,
leaf distribution, and shoot architecture. Tree growth is directly related to
the ability to intercept solar radiation (31,
32, 33). As trees grow, vertical foliage distribution generates
self-shading and reduces light to lower branches. This loss of light often
triggers crown recession, the shedding of shaded leaves, which strongly
influences growth dynamics (7, 15).
These conditions alter biomass partitioning among foliage,
branches, and trunk. After pruning, they also modify the relationship between
conductive tissue and leaf biomass (14). The Huber value
(Hv), defined as the ratio of xylem cross-sectional area (G) to total leaf
biomass (FB), is a key indicator of hydraulic function (23). Because gas
exchange occurs through the leaf surface, predicting biomass partitioning
requires considering both G and FB.
Silvicultural
practices modify this functional relationship. Pruning reduces active leaf
area, temporarily increasing Hv. This may enhance the ability of conductive
tissue to supply water to residual foliage but can cause short-term hydraulic
imbalance during drought (9, 21). In contrast,
thinning reduces competition and promotes both greater leaf area and conductive
tissue, thereby enhancing growth efficiency (11).
These responses
depend on treatment intensity, initial stand conditions, and resource
availability. To analyze them, we used linear mixed-effects models (MEMs). MEMs
decompose variability into components associated with treatments and site or
individual differences (4, 19, 35). They also handle
covariates effectively by adjusting for interactions and accounting for
dependencies such as repeated or nested data. This approach provides more
precise comparisons between treatments and controls, even in heterogeneous or
unbalanced datasets.
This study
evaluates the effects of pruning and thinning on aboveground biomass allocation
in Pinus ponderosa. We focus on the relationship between foliar biomass
and trunk growth, and how this relationship changes after treatment. By
analyzing biomass partitioning, we aim to determine whether silvicultural
practices alter the balance between foliage and conductive tissue, thereby
influencing growth dynamics and hydraulic function.
We hypothesize that
pruning reduces photosynthetic capacity by removing basal branches. This
reduction may decrease trunk growth and alter basal taper due to changes in
branch structure and radial growth. In contrast, thinning increases light
availability for remaining trees and reduces intraspecific competition. This
effect may compensate for foliage loss caused by pruning, favoring resource
allocation to trunk growth and potentially modifying xylem structure.
Given tracheid size
is a key determinant of hydraulic efficiency, we further hypothesize that
pruning and thinning induce adjustments in TL. These changes may represent
compensatory responses to altered canopy structure and resource availability.
If Hv values in
pruned and thinned trees converge toward those of controls, this would indicate
xylem adjustment to balance water transport and mechanical support. However, if
Hv differences persist, this would suggest long-term changes in biomass
allocation and a departure from the expected proportionality between conductive
tissue and foliage biomass.
These hypotheses
guide the assessment of whether pruned trees adjust hydraulic and mechanical
structures to maintain functional integrity under different management regimes.
Additionally, analyzing TL as an indicator of xylem plasticity, together with
wood density (WD), offers insight into how structural adjustments help trees
cope with changes in resource availability and canopy modification.
Materials
and Methods
We conducted a
completely randomized factorial design in a 12-year-old P. ponderosa plantation
in northwestern Chubut Province, Argentina (latitude -42.300059°, longitude
-71.296954°). The stand had a mean diameter at breast height (dbh) of 8.5 cm
and a mean top height of 4.43 m, with 3 × 3 m spacing. The site quality index
ranged from 13 to 15 m (1).
Four silvicultural
treatments were applied: pruning (P), thinning (T), pruning plus thinning (PT),
and a control (C). Each treatment was assigned to five experimental units
(EUs), for 20 units.
Each EU was a 144
m² plot with 16 trees, separated by a buffer row. Before applying treatments,
and again five years later, we measured all trees (n = 215). Measurements
included dbh with dendrometric tape, crown base height (CrwH) with metric tape,
and total height (TH) with a Haglöf Vertex III hypsometer. The dbh point was
permanently marked for consistent re-measurement. The crown base was defined as
the lowest whorl with at least three live branches, provided that all branches
below were dead or pruned.
Pruning removed 50%
of the basal crown. Thinning eliminated 50% of the trees, primarily smaller and
less vigorous individuals.
At the end of the experiment, we randomly selected 32 trees for
destructive sampling. Each treatment contributed eight trees, with at least two
per EU.
Vertical light
profiles were measured immediately after treatment and again five years later.
Eight HOBO sensors were mounted horizontally on a rod at 1 m intervals, from
0.3 m above ground to the apex. The top sensor served as the reference. The rod
was positioned at the crown periphery in four cardinal directions per tree for
at least one minute each time. Mean light intensity was then calculated per
tree, and integrated light intensity (ILI) along the crown was obtained using
Simpson’s rule (3).
Five years after
treatment, we felled the sampled trees and collected stem disks at stump height
(0.1 m) and at breast height (1.3 m). Polished and digitized discs were
analyzed with Map Maker v3.5 to measure total cross-sectional area (including
bark) and under-bark area. The latter represented woody tissues without
separating xylem and phloem. Bark proportion was also compared among treatments
and excluded from further analyses. Annual ring areas corresponding to the experimental
period were extracted to calculate cross-sectional area increment at both
heights, incGdbh and incGstump, respectively.
Tracheid length
(TL) was assessed in two annual rings per tree-one formed before and four years
after treatment. Wood samples corresponding to each ring were macerated
following the Franklin
(1937)
technique. Tracheids were measured under an optical microscope at 40×
magnification equipped with an ocular micrometer, following the anatomical
measurement standards of the IAWA (2004) and the
recommendations of Muñiz
and Coradin (1991). A total of 1,920 tracheids were measured (30 tracheids × 2
rings × 32 trees).
In addition,
oven-dried wood samples were used to determine anhydrous density at breast
height (2 annual rings × 32 trees; n = 64 samples). For this purpose, samples
were first saturated in water to determine their saturated weight, then
air-dried for 24 h, and subsequently oven-dried at 103°C to obtain anhydrous
weight. Between drying and weighing, samples were kept in a desiccator with
silica gel to prevent moisture absorption. Basic density was then calculated
using saturated and anhydrous weights, applying the maximum moisture content
formula described by Smith (1954).
We estimated total
tree foliar biomass (FBtree) in two steps. First, we developed an allometric
model predicting needle biomass from branch diameter (FBbranch), using 59 trees
from 15 regional plots.
These trees
represented a wide range of sizes (dbh: 5-38 cm; height: 3-21 m; crown length:
1.5-15.5 m; age: 9-34 years). One branch per tree was sampled. Twigs and
needles were separated, oven-dried at 60 °C, and weighed. The branch diameter
was measured 5 cm from the insertion using a digital caliper. In a second step
branch model was then applied to all branches of the sample trees to estimate
FBtree. Then we fitted a mixed-effects model (MEM) to predict FBtree using
“crown length × dbh²” as the main predictor. The model was validated with a
jackknife resampling procedure (5, 8).
We also tested
whether site quality (intercept growth, 1) was a significant covariate. The
allometric equation for branch biomass was: FBbranch [g] = 0.299 × dbh [mm]^2.186. Following Nakagawa and Schielzeth (2013), this model
explained 84% of the variance (marginal R² = 0.84). For FBtree, the marginal R²
was 0.859, with no significant effect of site quality. We therefore applied the
following equation to the factorial experiment: FBtree [kg] = 91.20 × crown
length [m] × dbh² [m²].
Statistical
analyses addressed the following variables: 1) incGdbh, 2) the relationship of
incGdbh vs Hv and FBtree, 3) the relationship of incGdbh vs incGstump, 4) TL,
5) WD, and 6) Hv.
We fitted MEMs for
variables 1), 2), 3), and 4) (table 1), and assessed fixed effects with likelihood ratio tests (LRT).
For all variables,
treatment differences were tested with Tukey-adjusted pairwise comparisons
using estimated marginal means (EMMs) (16). MEMs were adjusted according to Bates et
al. (2015). All analyses were performed in R (2021).
The general model
structure was:
y ∼ Fixed factors + Covariates + (1 | Grouping factor).
Specific cases for variables 1)- 3) are detailed in table 1.
Table 1. Full
MEMs formulations to perform tests. incGdbh: the
dependent cross-sectional area increments under bark at breast height.
Tabla
1. Descripción del modelo lineal
completo de efectos mixtos utilizados para los análisis. incGdbh:
variable dependiente, incremento del área transversal del tronco bajo la
corteza a la altura del pecho.
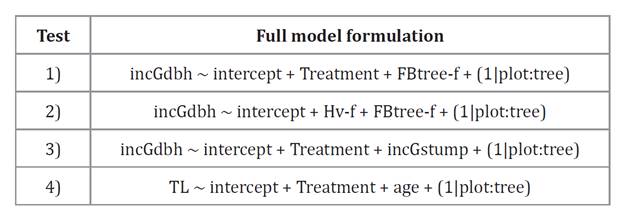
The
fixed effects factor was silvicultural treatment with levels C, P, PT, and T as
Treatment. Covariates FBtree-f: Tree foliar biomass at the end of the
experiment, Hv-f: Huber value at the end of the experiment, incGstump:
cross–sectional area increments under bark at stump height. Random effects:
grouping the individual tree nested in the EU, experimental unit, or plot.
El
factor de efectos fijos fue el tratamiento silvícola con niveles C, P, PT y T.
Covariables: FBtree-f: biomasa foliar del árbol al final del experimento; Hv-f:
valor de Huber al final del experimento; incGstump: incremento del área
transversal del tronco bajo la corteza a la altura del tocón. Efectos
aleatorios: agrupamiento del árbol individual anidado en la unidad experimental
(EU), o parcela.
Results
Bark proportion at
breast height did not differ among treatments (LRT; F = 0.582, df = 3, p =
0.633). Bark represented 19.7 ± 3.1% of trunk cross-sectional area. It was
excluded from all subsequent analyses.
At the beginning of
the experiment, integrated light intensity (ILI-i) (table 2) was higher in
pruned treatments P (90.4 ± 2%) and PT (90.1 ± 1%) than in control C (70.8 ±
2%) and thinning T (84.2 ± 1%). The similarity between P and PT indicates that
pruning was the main factor increasing crown light exposure, primarily by
raising crown base height (CrwH) (table 2).
Table 2. Tree
biometric values in the factorial experiment.
Tabla
2. Valores biométricos observados de
los árboles en el experimento factorial.
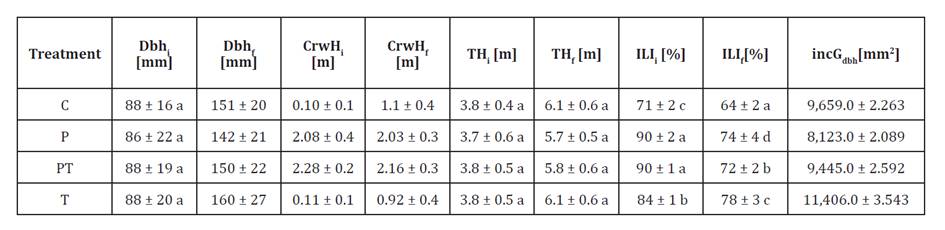
Mean
± standard deviation for each treatment. Dbh: diameter at 1.3 m height, CrwH:
live crown base height, TH: total height, ILI: integrated light intensity,
incGdbh: increment in cross-sectional area of woody tissues at breast height.
Suffixes “-i” and “-f” denote the initial and final moments of the experiment.
Different letters show significant statistical differences.
Media
± desviación estándar para cada tratamiento. Dbh: diámetro a 1,3 m de altura,
CrwH: altura de la base de la copa viva, TH: altura total, ILI: intensidad
lumínica integrada, incGdbh: incremento del área seccional de tejidos leñosos a
la altura del pecho. Los sufijos “i” y “f” indican los momentos inicial y final
del experimento. Letras distintas indican diferencias estadísticas
significativas.
By the end of the
experiment, integrated light intensity (ILI-f) decreased in all treatments: -
9.9% C,
-18.6% P, - 20.9% PT , - 7.5% T.
Despite this reduction, pruned treatments retained the highest final values-P
(73.6%) and PT (71.3%)-showing a lasting structural effect on canopy light
penetration.
Leaf biomass (FBtree) increased in all treatments during the
experiment (table
3).
The magnitude of change, however, differed among treatments. Control (C) and
thinning (T) reached the highest final values, 10.8 ± 3.8 kg and 12.6 ± 5.2 kg,
respectively. Both started from similar baselines, 2.8 ± 1.3 kg and 2.9 ± 1.7
kg, corresponding to increases of 287% and 331%.
Table 3. Functional
and structural values of tree traits in the factorial experiment.
Tabla
3. Valores funcionales y estructurales
observados de los atributos del árbol en el experimento factorial.
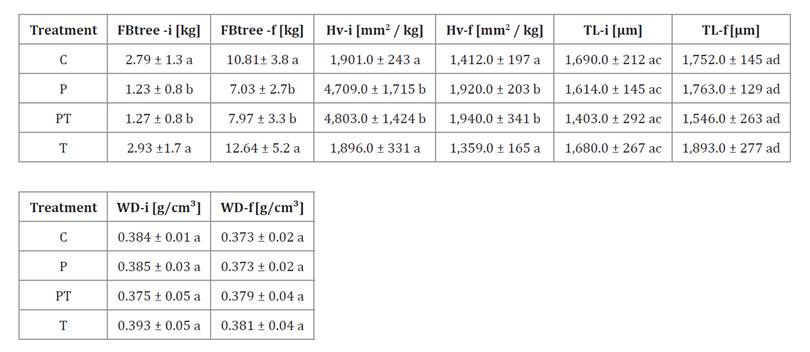
FBtree:
tree foliar biomass, Hv: Huber value, TL: tracheid length, and WD: wood
density. Mean ± standard deviation. Different letters show significant
statistical differences. For TL-i and TL-f, the first letter compares
treatment, and the second letter compares experimental moment - initial vs final-
for the same treatment. Suffixes “i” and “f” denote the initial and final
moments of the experiment.
FBtree:
biomasa foliar del árbol, Hv: valor de Huber, TL: longitud de traqueidas y WD:
densidad de la madera. Media ± desviación estándar. Letras distintas indican
diferencias estadísticas significativas. Para TL-i y TL-f, la primera letra
corresponde a la comparación entre tratamientos y la segunda al momento del
experimento -inicial vs final- para el mismo tratamiento. Los sufijos
“i” y “f” indican los momentos inicial y final del experimento.
In contrast,
pruning treatments began with significantly lower FBtree due to foliage
removal. Initial values were 1.2 ± 0.8 kg in P and 1.3 ± 0.8 kg in PT. By the
end, both reached intermediate levels: 7.0 ± 2.8 kg in P and 8.0 ± 3.3 kg in
PT. These increases of 472% and 528% indicate compensatory foliage regrowth in
pruned trees, while unpruned treatments followed steady canopy expansion.
Initial Hv-i (figure 1; table 3) was substantially
higher in P (4,709 ± 1,715 mm²/kg) and PT (4,803 ± 1,424 mm²/kg) than in C
(1,901 ± 243 mm²/kg) and T (1,896 ± 331 mm²/kg). This pattern reflected the
immediate pruning-induced reduction in leaf biomass. Over time, Hv declined in
all treatments, showing a rebalancing between conductive tissue and foliage.
The greatest declines occurred in P (-59.2%) and PT (-59.6%). Yet, both
treatments retained higher final Hv values than controls, indicating a
persistent structural effect of pruning.
Dots
jittered to provide a more comprehensive understanding.
Los
puntos están desplazados (jitter) para facilitar su visualización.
Figure
1. Huber values (Hv) for initial and final treatment
moments.
Figura
1. Valores observados de la razón de
Huber (Hv) al inicio y al final de los tratamientos.
Tracheid length
varied widely but increased in all treatments (figure 2), consistent with
age-related xylem maturation (Test 4 in table 1; AIC = 864.9,
model p = 0.0001, Age coefficient = 28.35, p = 0.003). Increases were largest
in T (+12.7%) and PT (+10.2%), followed by P (+9.2%) and C (+3.7%). These
results suggest that silvicultural treatments may accelerate tracheid
elongation. Although differences were not statistically significant, treatment
effects revealed biologically relevant trends.
Figure
2. Tracheid length distribution, before and after
treatment (C control, P Pruning, PT Pruning plus thinning, T thinning).
Figura
2. Distribución de la longitud de
traqueida, antes y después del tratamiento (C testigo, P poda, PT poda y raleo,
T raleo).
Wood density remained stable across treatments during the five
years (table
3).
Initial values ranged from 0.375 to 0.393 g/cm³. Final values showed only
slight variation (0.373-0.381 g/cm³). No significant differences were detected,
indicating that treatments did not markedly affect wood density.
In Test 1 (table
1;
figure
3),
the full model with treatments and FBtree-f explained trunk growth variation
(LRT: χ² = 422, df = 7, p < 2.2e-16). Predicted intercepts were highest for
PT (5,341.0 mm²), followed by P (4,492.0 mm²), T (4,013.0 mm²), and C (3,315.0
mm²). PT differed significantly from C (Δ = 1,626.0 ± 454 mm², p = 0.007). The
PT-T contrast approached significance (p = 0.076), suggesting a trend. A
complementary model using Hv-f and FBtree-f (Test 2) had similar explanatory
power (AIC = 3,556.5 vs. 3,547.8). This supports the hypothesis that
hydraulic adjustments mediate post-treatment growth.
Predictions
according to the full model of test 1.
Predicciones
según el modelo completo del test 1.
Figure
3. Observed (dots) and predicted (lines) values of
increment of cross-sectional trunk area (incGdbh) for treatments along tree
foliar biomass at the end of the experiment (FBtree-f).
Figura
3. Valores observados (puntos) y
predichos (líneas) del crecimiento del área transversal del tronco (incGdbh)
para los tratamientos, en función de la biomasa foliar final del árbol
(FBtree-f).
Finally, the
proportionality incGdbh vs incGstump, was not significantly affected by
treatments (Test 3, χ² = 1.6198, df = 3, p-value = 0.6549). Stem allocation
patterns, therefore, remained consistent despite pruning.
Discussion
Early silvicultural
treatments in Pinus ponderosa plantations produced clear changes in
growth and crown structure. Pruning and thinning, especially when combined,
enhanced trunk growth rates despite the initial reduction in foliar biomass.
Pruning increased crown light penetration, stabilized crown architecture, and
promoted compensatory foliage development.
These treatments also triggered structural and functional
adjustments. Pruned trees maintained higher Huber values (Hv) than controls
during the study period. Tracheid length increased across all treatments, with
greater elongation in treated trees. These anatomical shifts, although not
always linked to higher trunk growth, indicate xylem maturation adjustments
that may improve hydraulic efficiency.
The increased trunk
growth in the pruning plus thinning treatment (PT) supports the hypothesis that
P. ponderosa shows compensatory responses to early canopy interventions.
Despite foliage loss from pruning, treated trees -particularly under PT-
displayed greater xylem area increments, likely reallocating resources to
supportive structures. These dynamics align with compensatory growth theory,
which describes adaptive responses to sudden reductions in foliage (21,
22).
Our findings also
indicate that P. ponderosa adjusts hydraulic architecture without
compromising wood density. The separation between enhanced structural growth and
stable density suggests anatomical plasticity via tracheid elongation and crown
reconfiguration, not faster or lower-quality wood formation..
This result agrees with previous studies (9,
10, 11), which reported morphological and physiological adjustments
under pruning, thinning, and drought, including crown restructuring and
improved water-use efficiency, without changes in wood density. Likewise, Martínez-Meier
et al. (2015) detected fine-scale density variations with microdensitometry,
while our ring-level estimates revealed no significant effects, reinforcing the
idea of macro-anatomical rather than biochemical adaptation.
Although this study
focused on structural traits, the compensatory responses in trunk growth,
foliage regrowth, and xylem anatomy likely reflect ecophysiological
adjustments. Canopy opening in pruned treatments increased light exposure,
possibly enhancing stomatal conductance and photosynthetic rates. These changes
likely promoted carbon assimilation and foliage regeneration (20,
31).
Tracheid elongation
and shifts in Huber values further suggest adjustments in stem hydraulic
architecture. Such changes may improve specific hydraulic conductivity and
support water transport to the regenerating canopy (10,
13, 30). Reduced competition in thinned plots likely improved water
availability, favoring higher leaf water potential and maintaining stomatal
function (9, 11). Although not directly measured,
these responses match known mechanisms of resource reallocation and
water–carbon coupling in conifers under stress (15,
18).
These findings
refine the broader hypothesis by Fernández et al. (2011), who proposed that
Pinus species show lower physiological plasticity than Eucalyptus. While this
may hold at the biochemical level, our results highlight structural
adaptability in P. ponderosa. This species compensates for canopy
changes by adjusting conduit dimensions and crown structure to maintain
hydraulic function while keeping wood density stable. Such capacity has
important implications for resilience and productivity under silvicultural
management and environmental variability.
This study has
several limitations: a small sample size, a five-year monitoring period, and
the absence of direct physiological measurements. Another limitation is that
our design does not explicitly account for soil or landform heterogeneity,
which can modulate radial growth patterns in arid environments (27). Future work
should extend monitoring, include direct evaluations of stomatal conductance, photosynthesis,
and hydraulic conductivity, assess vascular reuse after pruning, and
incorporate spatial variation in site conditions. These efforts will help
clarify the functional mechanisms driving compensatory responses in P.
ponderosa.
Conclusions
This study confirms
that early pruning and thinning in Pinus ponderosa plantations trigger
compensatory growth, especially when both treatments are combined. The main
effects included greater conductive tissue area, partial recovery of foliar
biomass, and tracheid elongation, while wood density remained unchanged.
These structural adjustments support the hypothesis that
hydraulic and anatomical plasticity drive the observed responses. The findings
highlight the value of early silvicultural interventions to enhance growth and
maintain hydraulic function, providing guidance for management in temperate
conifer plantations. A deeper understanding of physiological and structural
adjustments will further inform strategies to optimize productivity and
resilience in P. ponderosa, especially in the early stages.
Acknowledgments
We thank the support staff of Campo Anexo San Martín – INTA for
assistance during sampling and Ea. El Maitén for providing the experimental
site. We are also grateful to the anonymous reviewers for their constructive
feedback, which improved the quality and rigor of the manuscript.
1. Andenmatten, E.;
Letourneau, F. J. 1997. Funciones de intercepción de crecimiento para
predicción de índice de sitio en pino ponderosa, de aplicación en la Región
Andino Patagónica de Río Negro y Chubut. Revista Quebracho. 5: 5-9.
2. Bates, D.;
Mächler, M.; Bolker, B.; Walker, S. 2015. Fitting Linear Mixed-Effects Models
Using lme4. Journal of Statistical Software. 67(1): 1-48. https://doi.org/10.18637/jss.v067.i01
3. Chapra, S. C.;
Canale, R. P. 2015. Numerical Methods for Engineers, 7th
ed. McGraw- Hill Education.
https://archive.org/details/numerical-methods-for-engineers-7th-edit/mode/2up
4. Demidenko, E.
2013. Mixed models: Theory and applications with R (2nd
ed.). John Wiley & Sons. (Wiley Series in Probability and
Statistics). p 717.
5. Fernández, M.
E.; Fernández Tschieder, E.; Letourneau, F. J.; Gyenge, J. E. 2011. Why do
Pinus species have different growth dominance patterns than Eucalyptus species?
A hypothesis based on differential physiological plasticity. Forest Ecology and
Management. 261(6): 1061-1068. https://doi.org/10.1016/j.foreco.2010.12.028
6. Franklin, G. L.
1937. Permanent preparations of macerated wood fibres. Tropical Woods. 49:
21-22.
7. Garber, S. M.;
Monserud, R. A.; Maguire, D. A. 2008. Crown recession patterns in three conifer
species of the Northern Rocky Mountains. Forest Science. 54(6): 633-646.
https://doi. org/10.1093/forestscience/54.6.633
8. Gregoire, T. G.
1984. The jackknife: A resampling technique with application to linear models.
Canadian Journal of Forest Research. 14(3): 483-487.
https://doi.org/10.1139/x84-092
9. Gyenge, J.;
Fernández, M. E.; Schlichter, T. 2009. Effect of pruning on branch production
and water relations in widely spaced ponderosa pines. Agroforest Syst. 77:
223-235. https://doi. org/10.1007/s10457-008-9183-9
10. Gyenge, J. E.;
Fernández, M. E.; Schlichter, T. M. 2010. Effect of stand density and pruning
on growth of ponderosa pines in NW Patagonia, Argentina. Agroforestry Systems.
78(3): 233-241. https://doi.org/10.1007/s10457-009-9240-z
11. Gyenge, J.;
Fernández, M. E.; Varela, S. A. 2012. Short-and long-term responses to seasonal
drought in ponderosa pines growing at different plantation densities in
Patagonia, South America. Trees. 26(6): 1905-1917.
https://doi.org/10.1007/s00468-012-0759-7
12. IAWA Committee.
2004. IAWA list of microscopic features for softwood identification. IAWA
Journal. 25(1): 1-70. https://doi.org/10.1163/22941932-90000349
13. Lachenbruch,
B.; McCulloh, K. A. 2014. Traits, properties, and performance: how woody plants
combine hydraulic and mechanical functions in a cell, tissue, or whole plant.
New Phytologist. 204(4): 747-764. https://doi.org/10.1111/nph.13035
14. Långström, B.;
Hellqvist, C. 1991. Effects of different pruning regimes on growth and sapwood
area of Scots pine. Forest Ecology and Management. 44: 239-254.
https://doi.org/10.1016/0378- 1127(91)90011-J
15. Ledermann, T.
2011. A non-linear model to predict crown recession of Norway spruce (Picea
abies [L.] Karst.) in Austria. Eur J Forest Res.
130: 521-531.
16. Lenth, R. V.
2024. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R package
version.
17. Letourneau, F.
J.; Medina, A. A.; Pampiglioni, A.; Ancalao, M.; Saihueque, M.; González, A.
2016. Efecto de tratamientos silvícolas sobre la maduración de la madera de una
plantación de Pino ponderosa. V Jornadas Forestales Patagónicas.
18. Li, C.; Barclay,
H.; Roitberg, B.; Lalonde, R. 2021. Ecology and Prediction of Compensatory
Growth: From Theory to Application in Forestry. Frontiers in Plant Science. 12.
https://doi. org/10.3389/fpls.2021.655417
19. Macêdo Araújo
da Silva, D.; Pereira da Silva Santos, N.; de Sousa Araújo Santos, E. E.; Alves
Pereira, G.; Barbosa da Silva Júnior, G.; Gomes da Cunha, J. 2022. Effects of
formative and production pruning on fig growth, phenology, and production.
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo.
Mendoza. Argentina. 54(1): 13-24. DOI: https:// doi.org/10.48162/rev.39.061
20. Martínez-Meier,
A.; Fernández, M. E.; Dalla-Salda, G.; Gyenge, J.; Licata, J.; Rozenberg, P.
2015. Ecophysiological basis of wood formation in ponderosa pine: Linking water
flux patterns with wood microdensity variables. Forest Ecology and Management.
346: 31-40. https:// doi.org/10.1016/j.foreco.2015.02.021
21. Maschinski, J.;
Whitham, T. G. 1989. The continuum of plant responses to herbivory: The
influence of plant association, nutrient availability, and timing. The American
Naturalist. 134(1): 1-19. https://doi.org/10.1086/284962
22. McNaughton, S.
J. 1983. Compensatory plant growth as a response to herbivory. Oikos. 40(3):
329-336. https://doi.org/10.2307/3544305
23. Mencuccini, M.;
Rosas, T.; Rowland, L.; Choat, B.; Cornelissen, H.; Jansen, S.; Kramer, K.;
Lapenis, A.; Manzoni, S.; Niinemets, Ü.; Reich, P. B.; Schrodt, F.;
Soudzilovskaia, N.; Wright, I. J.; Martínez-Vilalta, J. 2019. Leaf economics
and plant hydraulics drive leaf: wood area ratios. New Phytologist. 224(4):
1544-1556. https://doi.org/10.1111/nph.15998
24. Ministerio de Agroindustria. Unidad Para El Cambio
Rural-CIEFAP. 2017. Inventario de Plantaciones Forestales en Secano. Región
Patagonia. p. 136.
25. Muñiz, G.;
Coradin, V. 1991. Norma de procedimientos em estudios de anatomía de madeira.
II Gimnospermae. Brasília: Laboratorio de Produtos Florestais. Serie Técnica.
117 p.
26. Nakagawa, S.;
Schielzeth, H. 2013. A general and simple method for obtaining R² from
generalized linear mixed-effects models. Methods in Ecology and Evolution.
4(2): 133-142. https:// doi.org/10.1111/j.2041-210X.2012.00261.x
27. Piraino, S.;
Roig, F. A. 2024. Landform heterogeneity drives multi-stemmed Neltuma flexuosa
growth dynamics. Implication for the Central Monte Desert forest
management. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional
de Cuyo. Mendoza. Argentina. 56(1): 26-34. DOI:
https://doi.org/10.48162/rev.39.120
28. R Core Team.
2021. R: A language and environment for statistical computing. R Foundation for
Statistical Computing.
29. Smith, D. M.
1954. Maximum moisture content method for determining specific gravity of small
wood samples. Report N° 2014. USDA Forest Service. Forest Products Laboratory.
30. Sperry, J. S.;
Hacke, U. G.; Pittermann, J. 2006. Size and function in conifer tracheids and
angiosperm vessels. American Journal of Botany. 93(10): 1490-1500.
https://doi.org/10.3732/ ajb.93.10.1490
31. Wang, Y.; Liu,
Z.; Li, J.; Cao, X.; Lv, Y. 2024. Assessing the Relationship between Tree
Growth, Crown Size, and Neighboring Tree Species Diversity in Mixed Coniferous
and Broad Forests Using Crown Size Competition Indices. Forests. 15(633): 1-17.
https://doi.org/10.3390/ f15040633
32. Zeng, B. 2003.
Aboveground biomass partitioning and leaf development of Chinese subtropical
trees following pruning. Forest Ecology and Management 173: 135-144.
https://doi. org/10.1016/S0378-1127(01)00821-0
33. Zhu, Z.;
Kleinn, C.; Nölke, N. 2021. Assessing tree crown volume-a review. Forestry: An
International Journal of Forest Research. 94(1): 18-35.
https://doi.org/10.1093/forestry/cpaa037
34. Zingoni, M. I.;
Andia, I.; Mele, U. 2007. Longitud de traqueidas y madera juvenil en el fuste
de un árbol de pino ponderosa de 50 años-SO Neuquén. III Congreso
Iberoamericano de Productos Forestales IBEROMADERA 2007.
35. Zuur, A. F.; Ieno, E. N.; Walker, N. J.; Saveliev, A. A.;
Smith, G. M. 2009. Mixed effects models and extensions in ecology with R.
Springer Science & Business Media. https://doi.
org/10.1007/978-0-387-87458-6


