Fungicide Management of Late Leaf Spot and Peanut Smut
DOI:
https://doi.org/10.48162/rev.39.175Palabras clave:
Arachis hypogaea, control químico, enfermedades fúngicas, Nothopassalora personata, Thecaphora freziiResumen
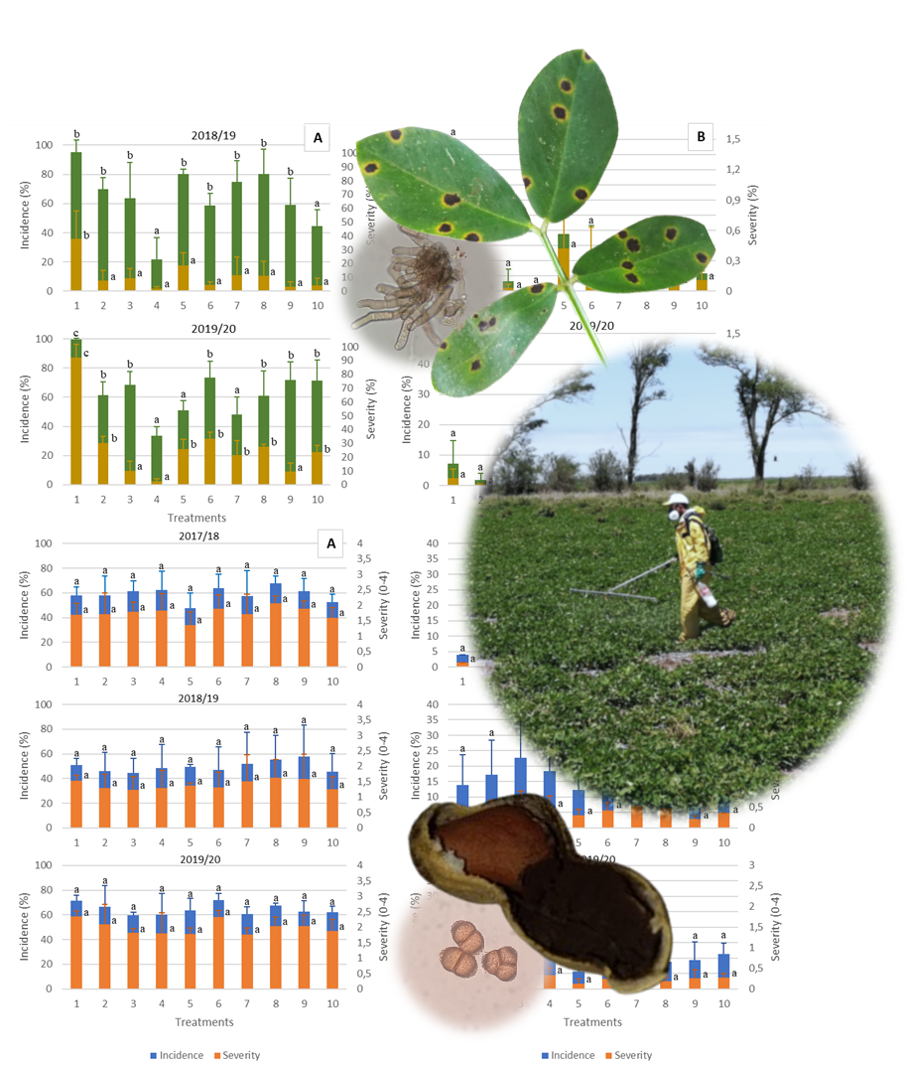
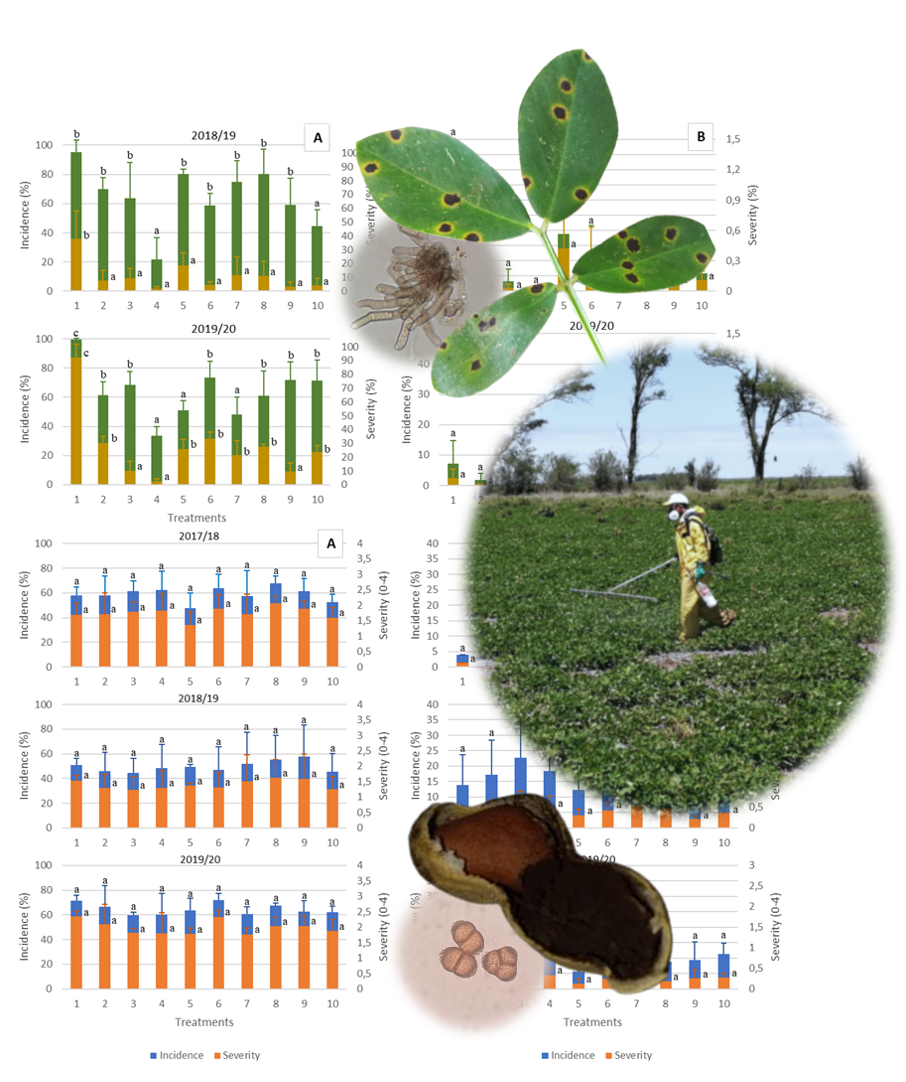
Late leaf spot (LLS), caused by Nothopassalora personata, is the most devastating peanut disease in the world. In Argentina, peanut smut (Thecaphora frezii) has increased significantly in recent decades. LLS is mainly managed through chemical fungicides, however, peanut smut is not effectively controlled, except for some resistant peanut genotypes. This study evaluated the effects of widely used fungicides for LLS control on both diseases and crop yield. Field trials were conducted over three consecutive years in two locations, with different fungicide doses and number of applications. Disease intensities were significantly higher in General Cabrera (GC) than in Vicuña Mackenna (VM) resulting in higher yields in VM. This could be due to the longer history of peanut cultivation in GC, where fungicide applications reduced LLS intensity. Among fungicides, chlorothalonil showed the best performance. However, these treatments were ineffective against peanut smut, likely due to difficulties reaching the infection site. Considering fungicides are one major management tool, further study of different active ingredients against both diseases should also consider sustainable integrated management.
Highlights:
- The intensity of late leaf spot and peanut smut was strongly associated with location.
- The use of chemical treatments proved effective in controlling late leaf spot.
- Peanut smut was not managed thought fungicides.
- It is possible to manage peanut late leaf spot using fungicides with a lower environmental impact.
Descargas
Citas
Amaradasa, B. S.; Everhart, S. E. 2016. Effects of sublethal fungicides on mutation rates and genomic variation in fungal plant pathogen, Sclerotinia sclerotiorum. PLoS One. 11(12): e0168079. https://doi.org/10.1371/journal.pone.0168079
Anco, D. J.; Thomas, J. S.; Jordan, D. L.; Shew, B. B.; Monfort, W. S.; Mehl, H. L.; Small, I. M.; Wright, D. L.; Tillman, B. L.; Dufault, N. S.; Hagan, A. K.; Campbell, H. L. 2020. Peanut yield loss in the presence of defoliation caused by late or early leaf spot. Plant Disease. 104: 1390-1399. https://doi.org/10.1094/PDIS-11-19-2286-RE
Augusto, J.; Brenneman, T. B. 2011. Implications of fungicide application timing and post-spray irrigation on disease control and peanut yield. Peanut Science. 38(1): 48-56. https://doi. org/10.3146/PS10-11.1
Augusto, J.; Brenneman, T. B.; Culbreath, A. K.; Sumner, P. 2010. Night spraying peanut fungicides. I. Extended fungicide residual and integrated disease management. Plant Disease. 94(6): 676-682. https://doi.org/10.1094/PDIS-94-6-0676
Bressano, M.; Massa, A.; Arias, R.; De Blas, F.; Oddino, C.; Faustinelli, P.; Soave, J.; Soave, S.; Perez, A.; Sololev, V.; Marshall, C.; Balzarini, M.; Buteler, M.; Seijo, G. 2019. Introgression of peanut smut resistance from landraces to elite peanut cultivars (Arachis hypogaea L.). PLoS ONE. 14(2): e0211920. https://doi.org/10.1371/journal.pone.0211920
Carmona, M.; Sautua, F.; Pérez-Hérnandez, O.; Reis, E. M. 2020. Role of fungicide applications on the integrated management of wheat stripe rust. Frontiers in Plant Science. 11: 733. https:// doi.org/10.3389/fpls.2020.00733
CASAFE. 2023. Cámara de Sanidad Agropecuaria y Fertilizantes. Guía online de productos fitosanitarios. https://guiaonline.casafe.org/ (Accessed on: Sep. 6 2023).
Cazón, I.; Bisonard, E. M.; Conforto, C.; March, G.; Rago, A. 2013. Estrategias para el manejo del carbón del maní. Actas de resúmenes XXVIII Jornada Nacional del Maní. General Cabrera, Córdoba. Argentina. p: 28-30.
Culbreath, A. K.; Gevens, A. J.; Stevenson, K. L. 2018. Relative effects of demethylation-inhibiting fungicides on late leaf spot of peanut. Plant Health Progress. 19(1): 23-26. https://doi. org/10.1094/PHP-09-17-0053-RS
Culbreath, A. K.; Brenneman, T. B.; Kemerait, R. C.; Stevenson, K. L.; Henn, A. 2020. Effect of DMI and QoI fungicides mixed with the SDHI fungicide penthiopyrad on late leaf spot of peanut. Crop Protection. 137: 105298. https://doi.org/10.1016/j.cropro.2020.105298
Dias, M. A. 2012. Phytotoxicity: An overview of the physiological responses of plants exposed to fungicides. Journal of Botany. Article ID 135479. https://doi.org/10.1155/2012/135479
Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; Gonzales, L.; Tablada, M.; Robledo, C. W. InfoStat versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Dugan, S. T.; Muhammetoglu, A.; Uslu, A. 2023. A combined approach for the estimation of groundwater leaching potential and environmental impacts of pesticides for agricultural lands. Science of The Total Environment. 901: 165892. https://doi.org/10.1016/j. scitotenv.2023.165892
Fulmer, A. M. 2017. Differentiation, prediction and management of early and late leaf spot of peanut in the southeastern United States and Haiti. Ph.D. thesis. University of Georgia, Athens, GA.
Ganuza, M.; Pastor, N.; Erazo, J.; Andrés, J.; Reynoso, M.; Rovera, M.; Torres, A. 2018. Efficacy of the biocontrol agent Trichoderma harzianum ITEM 3636 against peanut smut, an emergent disease caused by Thecaphora frezii. European Journal of Plant Pathology. 151(1): 257-262. https://doi.org/10.1007/s10658-017-1360-0
Giordano, D.F.; Pastor, N.; Palacios, S.; Oddino, C.; Torres, A. 2021. Peanut leaf spot caused by Nothopassalora personata. Tropical plant pathology. 46: 139-151. https://doi. org/10.1007/s40858-020-00411-3
Jordan, B. S.; Culbreath, A. K.; Brenneman, T. B.; Kemerait, R. C.; Branch, W. D. 2017. Late leaf spot severity and yield of new peanut breeding lines and cultivars grown without fungicides. Plant Disease. 101(11): 1843-1850. https://doi.org/10.1094/PDIS-02-17-0165-RE
Kovach, J.; Petzoldt, C.; Degni, J.; Tette, J. 1992. A method to measure the environmental impact of pesticides. New York’s Food and Life Sciences Bulletin. 139: 1-8.
Laboratorios NOVA. 2023. IRIDIUM. https://laboratorios-nova.com/fungicidas-fungicidas-insecticidas/iridium/ (Accessed on Sep. 12 2024).
Marinelli, A.; March, G.; Oddino, C. 2008. Aspectos biológicos y epidemiológicos del carbón del maní (Arachis hypogaea L.) causado por Thecaphora frezii Carranza & Lindquist. AgriScientia. 25(1): 1-5.
Marinelli, A.; Oddino, C.; March, G. 2017. 2ª ed. Enfermedades fúngicas del maní. En: Fernández, E.; Giayetto, O. (Ed.). El cultivo de maní en Argentina. Río Cuarto, Córdoba. Ediciones UNRC. p: 285-311.
Oddino, C.; Mortigliengo, S.; Moresi, A.; Soave, J.; Giuggia, J.; Ferrari, S.; Cassano, C.; Martinez, F.; Molineri, A.; Moran, F.; Soave, S.; Torre, D.; Butteler, M.; Bianco, C.; Bressano, M.; De Blas, F. 2017. Efecto de fungicidas foliares sobre la intensidad de viruela y carbón en diferentes cultivares de maní. Ciencia y tecnología de Cultivos Industriales. 6(9): 99-105.
Oddino, C.; Giordano, F.; Paredes, J.; Cazón, L.; Giuggia, J.; Rago, A. 2018. Efecto de nuevos fungicidas en el control de viruela del maní y el rendimiento del cultivo. Ab Intus. 1(1): 9-17.
Oddino, C.; Rosso, M.; Soave, J.; Soave, S.; Mendoza, M.; Giordano, D. F.; Bressano, M.; De Blas, F.; Mortigliengo, S.; Butteler, M. 2023. Comportamiento de variedades de maní resistentes a carbón a través de los años. Actas de resúmenes XXXVIII Jornada Nacional del Maní. General Cabrera, Córdoba. Argentina.
Paredes, J. 2017. Importancia regional del carbón del maní (Thecaphora frezii) y efecto de ingredientes activos de fungicidas sobre la intensidad de la enfermedad. Master thesis. Universidad Nacional de Río Cuarto, Córdoba.
Paredes, J. A.; Cazón, L. I.; Oddino, C.; Monguillot, J. H.; Rago, A. M.; Edwards Molina, J. P. 2021. Efficacy of fungicidal management of peanut smut. Crop Protection. 140: 105403. https:// doi.org/10.1016/j.cropro.2020.105403
Paredes, J. A.; Edwards Molina, J. P.; Cazón, L. I.; Asinari, F.; Monguillot, J. H.; Morichetti, S. A.; Rago, A. M.; Torres, A. M. 2022. Relationship between incidence and severity of peanut smut and its regional distribution in the main growing region of Argentina. Tropical Plant Pathology. 47: 233-244. https://doi.org/10.1007/s40858-021-00473-x
Paredes, J. A.; Guzzo, M. C.; Monguillot, J. H.; Asinari, F.; Posada, G. A.; Oddino, C. M.; Giordano, D. F.; Morichetti, S. A.; Torres, A. M.; Rago, A. M.; Monteoliva, M. I. 2024. Low water availability increases susceptibility to peanut smut (Thecaphora frezzii) in peanut crop. Plant Pathology. 73(2): 316-325. https://doi.org/10.1111/ppa.13810
Plaut, J. L.; Berger, R. D. 1980. Development of Cercosporidium personatum in three peanut canopy layers. Peanut Science. 7(1): 46-49. https://doi.org/10.3146/i0095-3679-7-1-11
Rago, A.; Cazón, I.; Paredes, J.; Edwards Molina, J.; Bisonard, M.; Oddino, C. 2017. Peanut Smut: From an emerging disease to an actual threat to Argentine peanut production. Plant Disease. 101(3): 400-408. http://dx.doi.org/10.1094/PDIS-09-16-1248-FE
Shokes, F. M.; Berger, R. D.; Smith, D. H.; Rasp, J. M. 1987. Reliabity of disease assessment procedures. A case study with late leafspot of peanut. Oléagineux. 42: 245-251.
USDA. 2023. United States Department of Agriculture. Peanut explorer. https:// ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2221000&sel_ year=2022&rankby=Production (Accessed on Sep. 12 2023).
Woodward, J. E.; Brenneman, T. B.; Kemerait, R. C.; Smith, N. B.; Culbreath, A. K.; Stevenson, K. L. 2008. Use of resistant cultivars and reduced fungicide programs to manage peanut diseases in irrigated and non-irrigated field. Plant Disease. 92(6): 896-902. http://dx.doi. org/10.1094/PDIS-92-6-0896
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2018 Revista de la Facultad de Ciencias Agrarias UNCuyo

Esta obra está bajo una licencia internacional Creative Commons Reconocimiento-NoComercial-CompartirIgual 3.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan las Políticas Editoriales.











.jpg)




