Essential Oils and Extracts from Argentinian Northwest Plants as Potential Biofungicides for Olive and Grapevine Pathogens: in vitro Studies
DOI:
https://doi.org/10.48162/rev.39.174Keywords:
Verticillium dahliae Kleb, Phaeoacremonium parasiticum (Ajello, Georg & C. J. K. Wang) W. Gams, Crous & M. J. Wingf, botanical antifungals, mycelial inhibition, conidial susceptibilityAbstract
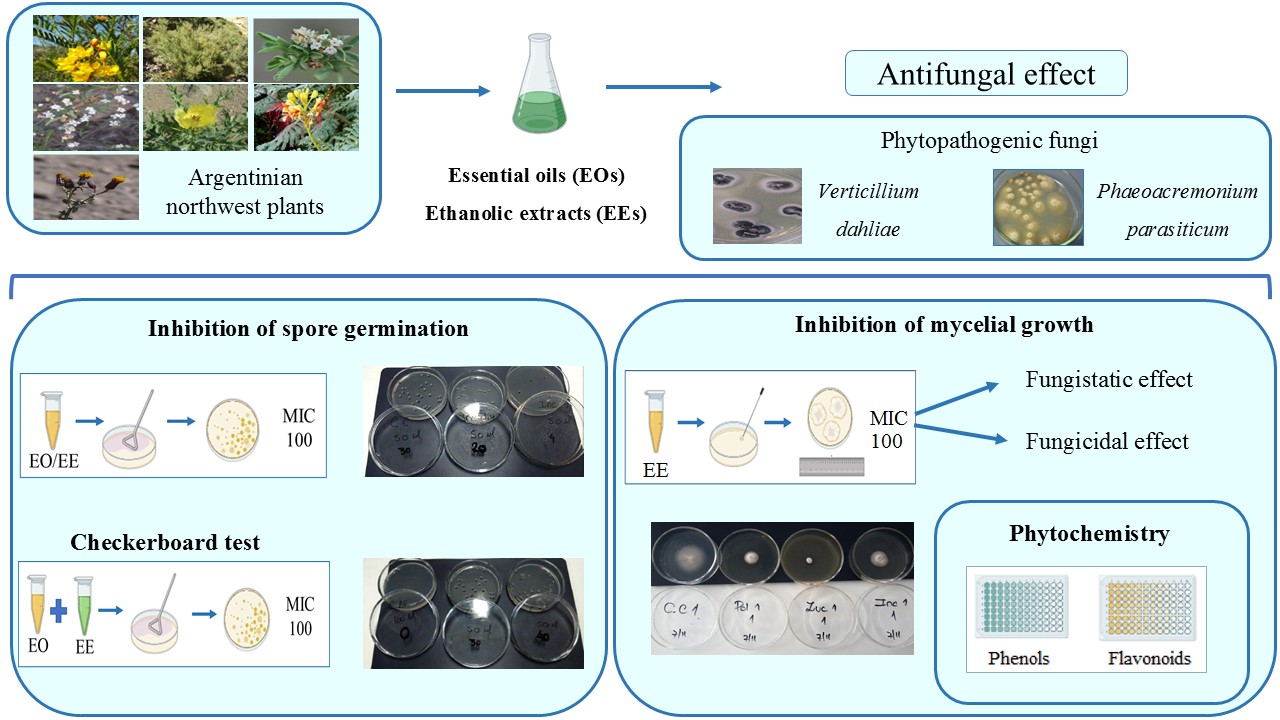
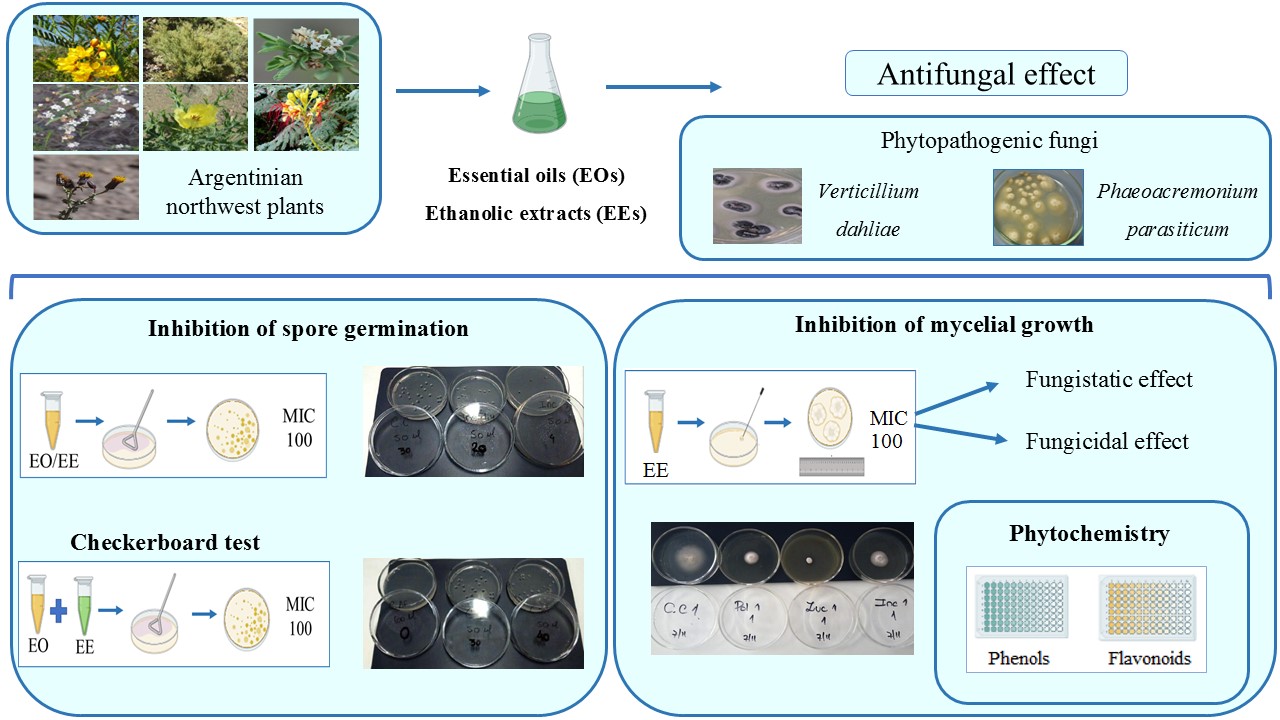
This work studies the effect of 12 botanical products from Argentinian northwest plants on spores and mycelium of Verticillium dahliae and Phaeoacremonium parasiticum, two pathogens of agronomic importance for the region. The fungi were exposed to essential oils (EOs) or ethanolic extracts (EEs), determining the percentage of germinated spores and mycelial growth. All tested EOs and EEs showed varying degrees of antifungal activity, dependent on plant species, extract type, pathogen, and targeted fungal structures. V. dahliae germination was completely inhibited by Zuccagnia punctata and Clinopodium gilliesii EOs. In experiments with EEs, Z. punctata EE was the most effective in suppressing spore germination of both fungi. The C. gilliesii EE also controlled V. dahliae germination. The EEs of Z. punctata, C. gilliesii and Lippia turbinata were the most active against mycelial growth. These three EEs had a fungistatic effect on P. parasiticum while Z. punctata and L. turbinata EEs showed a fungicidal effect on V. dahliae. The products obtained from Z. punctata, C. gilliesii and L. turbinata have potential as biocontrollers against V. dahliae and P. parasiticum. This is encouraging since no effective treatments are available for the diseases involving these pathogens.
Highlights:
- Antifungal activity of plant-derived products varied depending on plant species, extract type, pathogen, and targeted fungal structures.
- Ethanolic extracts (EEs) showed higher antifungal activity than Essential oils (EOs).
- V. dahliae was more sensitive to plant product activity than P. parasiticum.
- The studied botanical products offer promising eco-friendly alternatives for integrated disease management in regional crops.
Downloads
References
Alonso, J.; Desmarchelier, C. 2015. Plantas medicinales autóctonas de la Argentina. Corpus Libros Médicos y Científicos. Buenos Aires. 748 p.
Barbieri, N.; Gilabert, M.; Benavente, A. 2023. Phytochemical analysis and biological potential of Argentinian plant essential oils and extracts. Braz J Med Plants 25: 17-28.
Boiteux, J.; Fernández, M. de los Á.; Espino, M.; Silva, M. F.; Pizzuolo, P. H.; Lucero, G. S. 2023. In vitro and in vivo efficacy of Larrea divaricata extract for the management of Phytophthora palmivora in olive trees. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 55(2): 97-107. DOI: https://doi.org/10.48162/rev.39.112.
Di Liberto, M. G.; Stegmayer, M. I.; Fernández, L. N.; Quiroga, A. D.; Svetaz, L. A.; Derita, M. G. 2023. Control of brown rot produced by Monilinia fructicola in peaches using a full-spectrum extract of Zuccagnia punctata Cav. Horticulturae. 9(10): 1141. DOI: 10.3390/horticulturae9101141
Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.; Robledo, C. W. InfoStat versión 2020. Centro de Transferencia InfoStat, FCA. UNC. Córdoba. Argentina. http://www.infostat.com.ar
Đorđević, M.; Dolovac, N.; Ivanović, M.; Damnjanović, J.; Zečević, B. 2013. Effectiveness of essential oils in control of Verticillium dahliae in vitro. Zaštita bilja. 64(3): 162-168.
EPSA. 2023. Estrategia provincial para el sector agroalimentario. Provincia de La Rioja. Ministerio de Producción y Ambiente. www.argentina.gob.ar/sites/default/files/2023/05/la_rioja_2023.pdf
Erdogan, O.; Çelik, A.; Zeybek, A. 2016. In vitro antifungal activity of mint, thyme, lavender extracts and essential oils on Verticillium dahliae Kleb. Fresenius Environ Bull. 25: 4856-4862.
Escoriaza, G.; Sansberro, P.; Lampasona, S. G.; Gatica, M.; Piccoli, P. 2013. In vitro cultures of Vitis vinifera L. cv Chardonnay synthesize the phytoalexin nerolidol upon infection by Phaeoacremonium parasiticum. Phytopathol Mediterr. 52(2): 289-297.
Escoriaza, G.; García Lampasona, S.; Gomez Talquenca, S.; Piccoli, P. 2019. In vitro plants of Vitis vinifera respond to infection with the fungus Phaeoacremonium parasiticum by synthesizing the phytoalexin nerolidol. PCTOC. 138(3): 459-466. DOI: 10.1007/s11240-019-01641-3
Giamperi, L.; Fraternale, D.; Ricci, D. 2002. The in vitro action of essential oils on different organisms. J Essent Oil Res. 14(4): 312-318. DOI: 10.1080/10412905.2002.9699865
INV. 2023. Informe anual de superficie. 2022. Instituto Nacional de Vitivinicultura. www.argentina.gob.ar/inv/vinos/estadisticas/superficie/anuarios
Jimenez, C. M.; Sampietro, D. A.; Sgariglia, M. A.; Soberón, J. R.; Vattuone, M. A. 2014. Isolation, identification and usefulness of antifungal compounds from Zuccagnia punctata for control of toxigenic ear rot pathogens. Nat Prod Commun. 9(10): 1934578X1400901.
Kadoglidou, K.; Lagopodi, A.; Karamanoli, K.; Vokou, D.; Bardas, G.; Menexes, G.; Constantinidou, H. I.; Kadoglidou, K.; Karamanoli, K.; Lagopodi, A.; Bardas, G.; Vokou, D.; Menexes, G. 2011. Inhibitory and stimulatory effects of essential oils and individual monoterpenoids on growth and sporulation of four soil-borne fungal isolates of Aspergillus terreus, Fusarium oxysporum, Penicillium expansum, and Verticillium dahliae. Eur. J. Plant Pathol. 130. DOI: 10.1007/s10658-011-9754-x
Leal, L. E.; Alarcón, A. A.; Ortega-Baes, P.; Cayo, F.; Alarcón, R. 2018. Effects of essential oils from two Lippia species on growth of phytopathogenic fungi. BLACPMA. 17(1): 30-35.
Liu, J.; Hagberg, I.; Novitsky, L.; Hadj-Moussa, H.; Avis, T. J. 2014. Interaction of antimicrobial cyclic lipopeptides from Bacillus subtilis influences their effect on spore germination and membrane permeability in fungal plant pathogens. Fungal Biol. 118(11): 855-861. DOI: 10.1016/j.funbio.2014.07.004
Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. 2018. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 102(7): 1189-1217.
Montes-Osuna, N.; Mercado-Blanco, J. 2020. Verticillium wilt of olive and its control: What did we learn during the last decade? Plants. 9(6): 735. DOI: 10.3390/plants9060735
Mulero-Aparicio, A.; Varo, A.; Agustí-Brisach, C.; López-Escudero, F. J.; Trapero, A. 2020. Biological control of Verticillium wilt of olive in the field. Crop Protection 128: 104993. DOI: 10.1016/j.cropro.2019.104993
Rattalino, D. 2023. Identificación molecular y variabilidad genética de Verticillium dahliae su relación con la incidencia y prevalencia de la verticilosis del olivo en la zona olivícola de la provincia de La Rioja. Tesis de Doctorado en ciencias agropecuarias. Universidad Nacional de Córdoba. Argentina. 146 p.
Rattalino, D.; Otero, M. L.; Moriconi, D. N.; Rivera, P. C. 2021. Mejora de la detección del patotipo no defoliante de Verticillium dahliae en olivo mediante PCR anidada. AgriScientia. 38(1): 79-91. DOI: 10.31047/1668.298x.v38.n1.28985
Stegmayer, M. I.; Fernández, N. L.; Álvarez, N. H.; Olivella, L.; Gutiérrez, H. F.; Favaro, M. A.; Derita, M. G. 2021. Aceites esenciales provenientes de plantas nativas para el control de hongos fitopatógenos que afectan a frutales. FAVE. 20(1): 317-329. DOI: 10.14409/fa.v20i1.10273
Svetaz, L.; Tapia, A.; López, S. N.; Furlán, R. L. E.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S. A. 2004. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against Soybean Infecting Fungi. J. Agric. Food Chem. 52(11): 3297-3300. DOI: 10.1021/jf035213x
Varo, A.; Mulero-Aparicio, A.; Adem, M.; Roca, L. F.; Raya-Ortega, M. C.; López-Escudero, F. J.; Trapero, A. 2017. Screening water extracts and essential oils from Mediterranean plants against Verticillium dahliae in olive. Crop Protection. 92: 168-175. DOI: 10.1016/j. cropro.2016.10.018
Yadav, M. K.; Chae, S. W.; Im, G. J.; Chung, J. W.; Song, J. J. 2015. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. Plos One. 10(3): e0119564. DOI: 10.1371/journal.pone.0119564
Zaker, M. 2016. Natural plant products as eco-friendly fungicides for plant diseases control- A Review. The Agriculturists. 14(1): 134-141. DOI: 10.3329/agric.v14i1.29111
Zygadlo, J. A.; Grosso, N. R. 1995. Comparative study of the antifungal activity of essential oils from aromatic plants growing wild in the central region of Argentina. Flavour Fragr J. 10(2): 113-118. DOI: 10.1002/ffj.2730100210
Published
How to Cite
Issue
Section
License
Copyright (c) 2018 Revista de la Facultad de Ciencias Agrarias UNCuyo

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan las Políticas Editoriales.











.jpg)




